

As we continue to scale down electromechanical devices, we are approaching a discrete limit of nature. Traditional mechanisms fail at the scale of individual molecules, forcing us to look in other directions. Motors and actuators, in particular, are difficult to scale down.
Nature offers us one solution in the form of biological macromolecules. Cells contain a variety of proteins that can transform the chemical energy of ATP into mechanical energy.
F-ATPase
F-ATPase is a protein involved in the synthesis and hydrolysis (breaking down) of ATP, the primary chemical energy source in organisms. Its diameter is around 12 nm, ideal for nanoscale electromechanical devices, and its structure resembles a six-lobed pumpkin with a long stalk. In the hydrolysis of ATP, this stalk rotates anti-clockwise at a no-load speed of 17 revolutions per second and is capable of exerting a force of greater than 100 pN.[1]
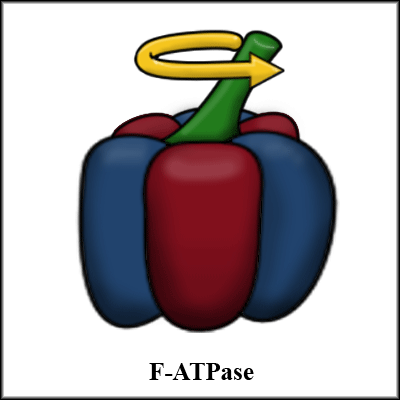
Figure 1
To fabricate a motor out of F-ATPase, Montemagno et al. genetically engineered the F-ATPase protein to express the His codon, allowing its base to bind to certain metals. By controlling the deposition of the metal, they could precisely position the motor on a substrate. Also, the tip of rotating stalk of the genetically engineered protein can selectively bond to certain materials. In this experiment, they used biotin-streptavidin binding to attach a fluorescent sphere to the tip of the stalk in order to measure its rotational speed using differential optical interferometry.[1]
Liu et al further engineered the F-ATPase protein to allow chemical switching of the motor, so that the presence of certain ions inhibits its function.[2] This sort of switching ability is necessary for integration into electromechanical devices.
Kinesin Conveyer Belts
Kinesin is a protein involved in intracellular transport, moving along microtubules found inside the cell. It is approximately 50 nm in size can exert 6 pN of force.[3]
Since each protein can only exert so little force, researchers generally immobilize the kinesin proteins in a channel so that the kinesin pushes the microtubules along the channel.[4] One issue that comes up, however, is that even though we can align the kinesin along a line, the direction of the motion depends on the polarity of the microtubules, making unidirectional flow impossible without a means of fixing the alignment of microtubules.
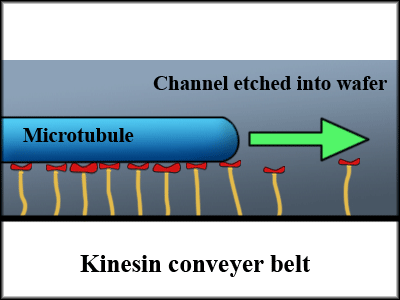
Figure 2
One way around this is to alter the geometry of the channels so that the microtubules are predominantly oriented in one particular direction. Clemmens et al created junctions more resembling city planning than the cutting edge of nanotechnology, but these were effective in determining microtubule flow.[5]
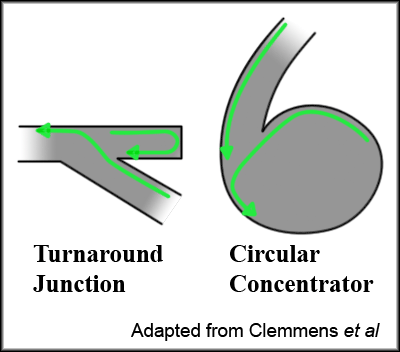
Figure 3, adapted from Clemmens et al.[5]
Finally, to utilize these proteins in an effective molecular conveyer belt, we need to be able to load and unload “cargo” from the microtubules and to control the motion. Hess et al used the same biotin-streptavidin binding that we saw used to bind fluorescent spheres to the stalk of the F-ATPase protein to bind fluorescent spheres to a biotin-coated microtubule. Their observations showed that moving microtubules would pick up any fluorospheres it came across in its path and randomly drop them off after transporting them along the channel.
Starting the kinesin conveyer belt is simply a matter of providing fuel in the form of ATP, but stopping it is a little more difficult. The kinesin proteins require so little energy that it the conveyer belt will run for a few hours just on the ATP in the channel. Hess et al addressed this issue by adding an ATP-consuming enzyme to the solution, decreasing this time down to a couple minutes. In addition, they bound the ATP in photoactive “cages” that released the ATP upon exposure to UV light. Thus, by simply varying the length of a UV light pulse, they could move the microtubules a certain distance and stop.[6]
Bio-NEMS
Nanoelectromechanical systems (NEMS) are constrained by the discreteness of the molecules they're built of, so ideally its functional parts are molecules themselves. Biology has offered one such solution in the form of proteins, and already researchers have integrated them into hybrid systems of biological and inorganic parts. It may be that we will one day be able to fabricate functional molecules, but until then the pre-made structures offered by biology can perform many of the tasks we need, and we will probably see many hybrid Bio-NEMS in the future.