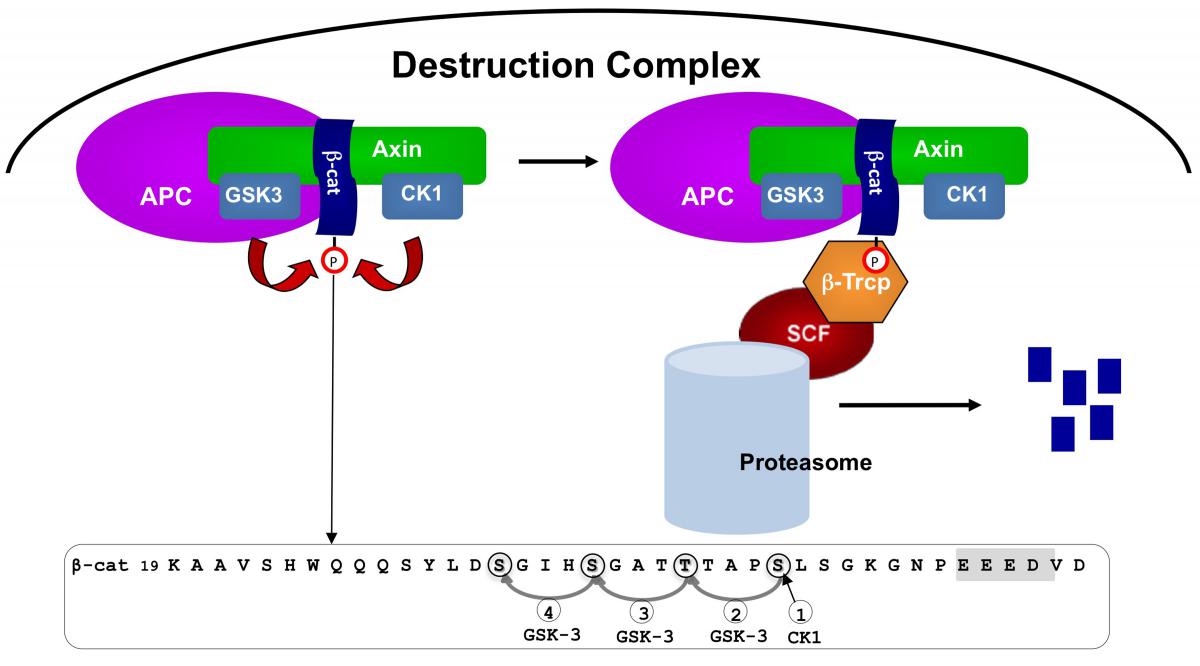
The ß-catenin destruction complex
Overview of the ß-catenin destruction complex
In the absence of a Wnt signal, ß-catenin is degraded by a complex of proteins including Axin, APC, the Ser/Thr kinases GSK-3 and CK1, protein phosphatase 2A (PP2A), and the E3-ubiquitin ligase b-TrCP. The complex specifies a ß-TrCP recognition site on ß-catenin by phosphorylation of a conserved Ser/Thr-rich sequence near the amino terminus. Phosphorylation requires scaffolding of GSK-3 and CK1 and ß- catenin by Axin. Aftter phosphorylation and ubiquitination, ß-catenin is degraded by the proteasome. In the figure, the phosphorylated sequence near the amino terminus of ß- catenin is shown, with CK1 and GSK-3 sites circled. The acidic cluster that primes CK1 phosphorylation is highlighted in gray. SCF, the Skp1/cullin/F-box complex. (From Stamos and Weis, 2012)