Drug Profile: Interferon-a
What is it?
IFN-a has been used as a treatment for chronic Hepatitis C since its approval in 1991. There are three types IFN-a2a, IFN-a2b, and IFN-an1. A combination of IFN and ribavirin has been shown to be more effective that either IFN or ribavirin alone. 49% of patients taking IFN-a2b and ribavirin showed a sustained biologic and virologic response, versus 5% of patients who were taking IFN-a2b alone. Roche produces Roferon-A-A and Schering-Plough makes Intron A, both IFN-a2b.
How does it work?
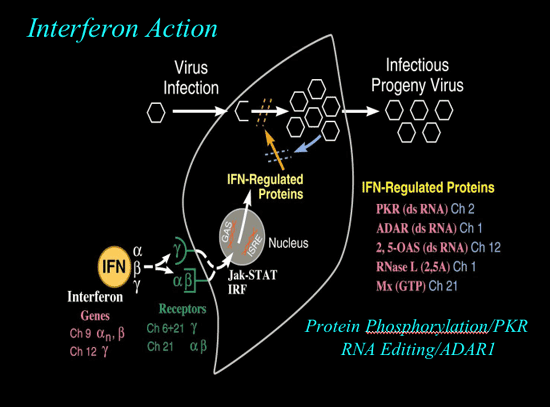
Interferons are naturally occurring proteins that have antiviral, antiproliferative, and immunomodulatory properties. Their efficacy in fighting hepatitis C lies in their ability to activate interferon-stimulating genes (ISGs), which prevent viral replication and alert the immune system.
Who should take it?
IFN treatment is only recommended for people with chronic heptatitis C who are at risk for progression to cirrhosis. This is evidenced by constantly elevated ALT (alanine transferase) levels, a liver biopsy of portal or bridging fibrosis and inflammation and necrosis. People with genotype 1 hepatitis C virus have been shown to respond better to treatment than those with genotypes 2 or 3. This suggests that patients should have their virus genotyped before beginning treatment.
Who should not take it?
This drug is not recommended for use in people under the age of 18 or those with histories of depression, cytopenia, hpothyroidism, or renal transplantation.
Dosage Recommendations:
3 million IU sc 3 times weekly + oral ribavirin 1200 mg daily (Merck)
Side effects:
Early: flu-like illness, chills, fever, malaise, myalgia, headache, poor appetite
Later (common): weight loss, increased need for sleep, irritability, anxiety, depression, hair loss, thrombocytopenia
Unusual or severe: seizures, acute psychosis, bacterial infections, autoimmune reactions, hyper or hypothroidism
Rare: proteinuria, myocardiopathy, rashes, intersitial lung disease, retinal changes, ototoxicity
New Developments:
Standard IFN is now being replaced by pegylated IFN. This new drug requires injection only once a week instead of three times a week. This is possible because one or more chains of polyethelene glycol (PEG) are bonded to each interferon molecule, keeping the drug in the body longer. The FDA has recently approved Schering-Plough's product, PEG-INTRON and Roche's Pegasys. Treatment with either of these drugs should only be initiated in people who have not been previously treated with IFN-a. The side effects are similar to those associated with standard IFN.
 Click here to learn more about Pegasys
Click here to learn more about Pegasys
 Click here to learn more about PEG-INTRON
Click here to learn more about PEG-INTRON
Sources:
Knipe and Howley, eds. Fields Virology, Fourth Edition. Lippincott, Williams, & Wilkins, 2001.
liverfoundation.org