Cystic Fibrosis References
| 1938-1967 | 1968-1996 | 1997-2002 |
|
Cystic Fibrosis References
|
| ..Cystic fibrosis (CF), is one of the more thoroughly understood genetic diseases. [FIG 1] As such, it provides guidance in understanding how a single genetic mistake can give rise to a wide array of medical problems. It also provides a cautionary tale: our ability to understand genes, proteins and even cells far exceeds our ability to unravel the complex interactions that give rise to organ-level disease. | 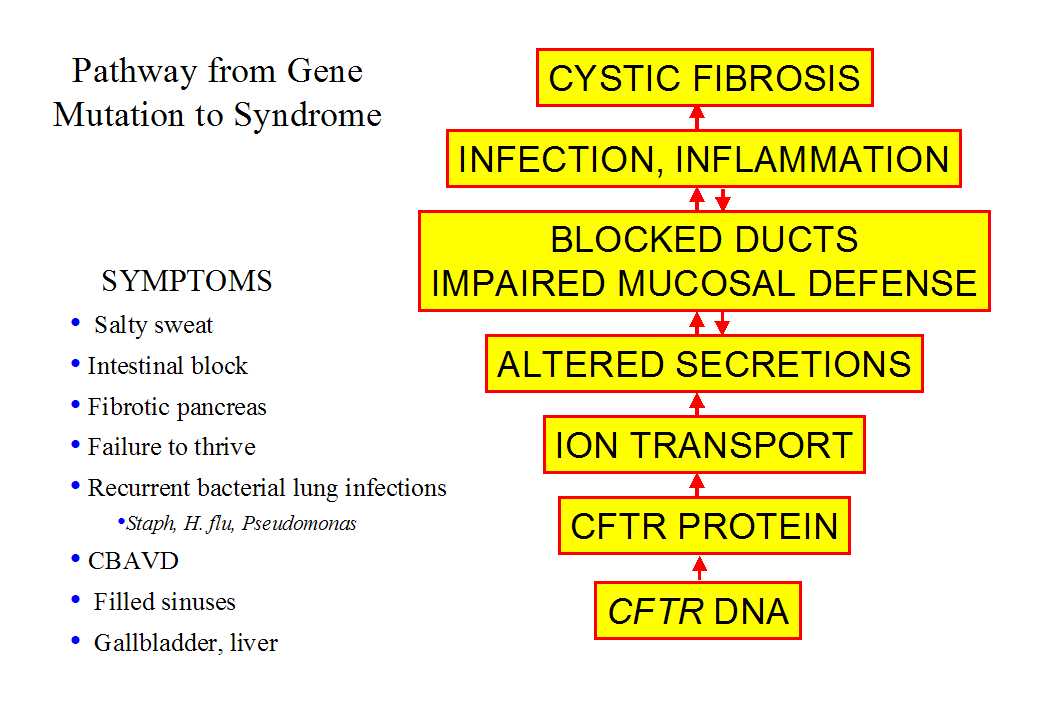 |
| It has been known for more than 50 years that cystic
fibrosis is an autosomal genetic disease (i.e. not sex linked) that causes
an accumulation of mucus in many exocrine and exocrine-associated organs,
but it is only in the last decade that the basis of CF was traced to defects
in CFTR
(also called ABCC7), a large gene on chromosome 7.
In populations of Northern European extraction, at least one person in
25 carries a mutation in CFTR. Rates decline geographically,
being moderate in Southern Europeans and lowest in Asian populations, who
have carrier rates of ~1/500.
The CFTR gene produces the protein CFTR, a member of the ATP Binding Cassette (ABC) family. ABC family members perform several tasks, including energy-dependent transmembrane transport of large molecules, regulation of other transmembrane transporters, and the transport of ions across membranes. CFTR displays at least two of these functions. It is an anion channel that uses the energy of ATP hydrolysis to transit through conducting and non-conducting conformations. Ion channels are equally adept at allowing ions to flow either into or out of cells, enabling the CFTR anion channel to play crucial roles in both absorption and secretion. CFTR is found primarily in wet epithelia, consistent with the symptoms that define CF. CFTR has the structure shown in the cartoon. It has 12 transmembrane spanning domains, two nucleotide binding domains, and a regulatory domain in the center. |
 |
It is apparent from a comparison of the intestine and sweat duct that lost CFTR function has different consequences depending upon the cellular context. This view is reinforced by considering other exocrine organs in CF humans and CF mice.
In summary, comparison of epithelial transport across various exocrine organs of humans and mice reveals a consistent general picture: after loss of CFTR, transport is abolished in epithelia that rely on CFTR for anion conductance and is spared in epithelia that have alternative channels. If secretions contain a high level of solids the lumen of the exocrine organ can be blocked, with severe consequences.
Given that so much else is understood about CF, it is frustrating that we still can’t explain CF lung disease. One difficulty is that lung disease, unlike CF disease in any other organ, is dominated by infection and inflammation which obscure the earliest steps in the disease process. The causal chain from CFTR mutations to lung disease is also obscure because even normal lungs are prone to infection. Lung diseases are the third most prevalent cause of fatal illness in the U.S. and are the most common cause of death of babies under age one. Thus even relatively minor decreases in airway defenses might tip the balance toward self-reinforcing infection. In normal airways, a complex hierarchy of defenses usually maintains sterility below the first bronchial division, despite constant inhalation of viruses, bacteria and mold spores. Several researchers, including Richard Boucher of UNC, have suggested that CFTR mutations impair not one but rather several aspects of the lung defense mechanisms. This combination of features would make analysis difficult even if we knew exactly how airway sterility is maintained in normal lungs. But we don't that, although we do understand the process in outline.
The airway surfaces are covered with a thin film (c. 30 mm deep) of airway surface liquid (ASL) consisting of a periciliary sol and a mucus gel that are propelled toward the mouth by coordinated ciliary beating. Thus mucociliary clearance, aided by cough, cleans the airways mechanically. ASL is not simply saltwater, but is instead a rich broth of proteases/antiproteases, oxidants/antioxidants, antibiotics, and antibodies that work together to inactivate or destroy pathogens without undue collateral damage to the lungs. These mucosal mechanisms are backstopped by cellular immune mechanismsthat are recruited and coordinated by signaling molecules released into the ASL.
The aggregate features of CF lung disease distinguish it from all other lung diseases. Like chronic bronchitis, CF affects the conducting airways and not the alveoli, and these two diseases are also unusual in that both affect females more severely than males. In CF, infections of the conducting airways are nearly universal, difficult and eventually impossible to eradicate, and ultimately lethal. The types of organisms that affect CF patients, such as Pseudomonas aeruginosa, Burkholderia cepacia, and Aspergillus are innocuous in normal individuals. Disease is most severe in the upper lobes and involves huge infiltrations of neutrophils.
Three hypotheses attempt to explain CF lung disease using
transport properties of CFTR (Wine,
1999)![]() .
Two
competing hypotheses consider CFTR's role in salt absorption to be primary,
while a third emphasizes CFTR’s role in secretion and is compatible with
either of the absorption hypotheses.
.
Two
competing hypotheses consider CFTR's role in salt absorption to be primary,
while a third emphasizes CFTR’s role in secretion and is compatible with
either of the absorption hypotheses.
The high salt hypothesis![]() ,
proposed by Jeffrey Smith, Michael Welsh and their colleagues at the University
of Iowa, emphasizes CFTR's function as an anion channel. According to this
hypothesis, the surface liquid of normal airways has low levels of salt
(less than half the value of plasma) because salt is absorbed in excess
of water. In CF airways, missing or defective CFTR causes reduced transepithelial
Cl- conductance, and
by analogy with the sweat duct this causes salt levels in the airway surface
liquid to remain at levels similar to those in plasma. The relevance of
"high salt" (my term) to CF lung disease is that it interferes with bacterial
killing by natural antibiotics such as defensins and lysozyme. Many of
these compounds interact electrostatically with the bacterial cell wall
and this interaction is sensitive to the ionic strength of the medium.
,
proposed by Jeffrey Smith, Michael Welsh and their colleagues at the University
of Iowa, emphasizes CFTR's function as an anion channel. According to this
hypothesis, the surface liquid of normal airways has low levels of salt
(less than half the value of plasma) because salt is absorbed in excess
of water. In CF airways, missing or defective CFTR causes reduced transepithelial
Cl- conductance, and
by analogy with the sweat duct this causes salt levels in the airway surface
liquid to remain at levels similar to those in plasma. The relevance of
"high salt" (my term) to CF lung disease is that it interferes with bacterial
killing by natural antibiotics such as defensins and lysozyme. Many of
these compounds interact electrostatically with the bacterial cell wall
and this interaction is sensitive to the ionic strength of the medium.
The high salt hypothesis is based on meticulous experiments in which airway epithelial cells from normal and CF individuals were grown on filters. Bacteria were then placed into the fluid covering the apical surfaces of the cultured cells. The remarkable finding was that bacteria flourished in the cultures of CF airway cells, but were killed in the cultures of normal cells! Bacteria placed in the media beneath normal cells flourished, suggesting that normal cultured airway cells produce apical factors that kill bacteria. A further remarkable discovery was made when the salt content of the apical fluid was manipulated. Merely adding pure water to the fluid from CF cells rendered it bactericidal while adding NaCl to fluid from normal cells allowed bacteria to grow! The Iowa group concluded that both normal and CF airway epithelial cells release antibiotics into the surface liquid, but in CF cultures the antibiotics are rendered ineffective by a higher salt concentration.
The reduced salt concentration postulated for normal airway surface fluid requires that salt be absorbed in excess of water, as it is in sweat ducts. However, because airway epithelia are more water permeable than ducts, additional osmotic forces are required so that the osmolarity of the surface liquid equals plasma values. What substitutes for the absorbed NaCl? Two possible sources for these forces have been postulated: surface tension created by the cilia or additional osmolytes that become important as the layer of surface fluid thins. Because it is difficult to measure airway surface fluid in vivo, the Iowa group developed a radioisotopic method to measure salt levels in the tiny volumes of surface fluid in their air interface cultures. As predicted, they found reduced salt levels in apical fluid covering normal cells, while apical fluid covering CF cells was ~twice as salty as normal.
Based on extensive experiments using several different
methods, Richard Boucher and his colleagues at the University of North
Carolina, came to a markedly different conclusion. Their "low volume" hypothesis,
(my term) is not based on CFTR's function as a channel. Instead, it is
based on CFTR’s ability to inhibit the activity of the epithelial sodium
channel (ENaC). According to this hypothesis, both normal and CF ASL have
plasma-like levels of salt, but in CF, CFTR's inhibition of ENaC is eliminated,
resulting in increased Na+
transport. Because significant Cl-
shunt pathways exist in the airways, increased Na+
transport drives increased absorption of Cl-
and water. Thus CF airways display accelerated isotonic fluid absorption
that depletes ASL volume and dehydrates mucus, leading to obstruction and
infection![]() .
.
To test the hypothesis they also grew airway epithelial cell cultures on filters, but unlike the Iowa group they found no difference in either the salt content or the osmolarity of surface liquid from normal and CF airway cultures, both of which were essentially equivalent to plasma. However, the liquid layer in their CF epithelial cultures was reduced in volume. To quantify hyperabsorption in CF airway cultures a 30 mm deep layer of fluid was followed over a 24 hour period, during which it was reduced to ~10 m m with no increase in Cl- concentration. These cultures secreted mucus, and after addition of fluid about 30% of the cultures showed regions of rotational mucus transport. Mucus movement continued in normal cultures, but stopped after 24 hours in CF cultures. Microscopic inspection showed that depletion of the sol layer in CF cultures collapsed the mucus layer onto the cilia and stopped transport. The UNC group assayed the water permeability of their human airway cultures by measuring changes over time in the osmolality and volume of hypotonic saline samples placed onto the apical surface. The hypotonic samples rapidly equilibrated to isotonicity as water shifted to the cellular and subcellular compartments. Additional experiments indicated that airway cells absorb isotonic fluid via a Na+-dependent mechanism because absorption stops when Na+-is replaced with an impermeant ion.
These findings fit with their extensive prior evidence of Na+ hyperabsorption in CF epithelia, and provide direct evidence for one of the earliest hypotheses to explain CF disease, the "thick mucus" hypothesis (CF is known as mucoviscidosis outside of the U.S.). If this happens in CF airways it should compromise mucociliary clearance and promote infection.
This hypothesis, also called the "serous cell malfunction" hypothesis, is based on CFTR’s role in fluid secretion. According to this hypothesis, human serous cells require CFTR for the secretion of the antibiotic-rich fluid that is elaborated by small airways and submucosal glands in response to irritants. In CF, fluid secretion by these cells is greatly diminished, resulting in airway mucus that is thicker and deficient in antibiotics. This hypothesized malfunction would exacerbate the defects proposed in each of the previous hypotheses: natural antibiotics would be not only less efficient but less abundant, and depletion of the airway surface liquid in CF would result from both decreased secretion and increased absorption.
| The serous cell malfunction hypothesis is based
on experiments by Walter Finkbeiner and Jonathan Widdicombe and their colleagues
of the University of California at San Francisco, Stephen Ballard and colleagues
at the University of South Alabama, John Engelhardt and his colleagues
at the University of Pennsylvania, and by my colleagues and me at Stanford.
The hypothesis begins with the observation that most symptoms of CF, such
as meconium ileus, loss of pancreatic function, degeneration of the vas
deferens, and thickened cervical mucus are attributed to CFTR's role in
Cl--driven fluid secretion;
we propose that CF lung disease conforms to this general pattern. In human
upper airways fluid secretion is primarily a function of the glands while
the surface epithelium is primarily absorptive. The highest levels of CFTR
occur in serous cells of submucosal glands. Gland serous cells are abundant
sources of bactericidal and antifungal compounds and are strategically
located in the gland acini to provide the liquid needed to hydrate mucins
released by mucous cells in the gland tubules.
Cell culture models of serous cells, comparison of primary cultures of normal and human gland cells, and experiments with airway glands in animal models all indicate that CFTR is critical for glandular fluid secretion. A similar CFTR-mediated fluid and secretion process is postulated to occur in distal airways, where CFTR-containing serous cells appear to be scattered on the surface of airway tubules. It seems evident that the loss of gland function will exacerbate whatever absorptive defect is eventually found. |
 |
Competing hypotheses co-exist only briefly unless methods are inadequate to decide among them. It might seem odd that there could be a disagreement about something as simple as the composition of airway surface liquid in human airways. However, there is no consensus on this point because the volume of fluid to be sampled is tiny and is rapidly altered when disturbed. Airway surface liquid has been estimated to be anywhere from 20-150 microns in depth depending upon species and method. The sol layer is usually about the same depth as the cilia, or approximately 10 microns. If a typical thickness is taken as 30 microns, then a square cm area would contain only 3 microliters, and the entire surface of the conducting airways, an area about 2 meters square, would be bathed in 60 milliliters or 4 tablespoons of fluid.
Is it possible that all three hypotheses are at least partially correct? The serous cell secretion hypothesis is compatible with either of the malabsorption hypotheses. In their simplest formulations the two absorption hypotheses differ beyond reconciliation, but we have learned that for biological mechanisms, simple usually means simplistic. Absorption of airway fluid may well be (at least) a two-stage process. When volume is high, the osmolarity of the solution is dominated by permeant electrolytes and iso-osmotic volume absorption dominates. As the surface layer is depleted, the contribution of factors such as ciliary surface tension or impermeant osmolytes increases, allowing depletion of permeant osmolytes. This explanation was proposed in 1995 by JH and JG Widdicombe, who suggested that ciliary surface tension supplied the required osmotic pressure. But to explain a salt difference between normal and CF airway surface liquid, it seems necessary to propose that the alternate (shunt) pathway for Cl- be closed as the fluid layer thins. This proposal will be difficult to evaluate experimentally.
Insights produced by these studies are spawning innovative
treatments for CF lung disease. The
high salt and serous cell malfunction hypotheses are focusing attention
onto the lung’s natural antibiotics, just when the idea of using inhaled
antibiotics is receiving powerful validation from large-scale trials. The
low volume and serous cell malfunction hypotheses are rekindling efforts
to increase the fluidity and mobility of lung secretions. The increased
effectiveness of inhaled vs. IV antibiotics, the formulation of additional
inhaled antibiotics to prevent the development of bacterial resistance,
the invention of highly efficient nebulizers, and early treatment of lung
inflammation (eventually through inhalation) promise, when used in combination,
to prolong the lives of CF patients. Crucial to these efforts is a change
in outlook toward treating the lungs continuously before infections
become entrenched. That’s what normal lungs to do naturally, and we are
finally following nature’s lead.
| Wine |