Juvenile Huntington’s Disease (Text and Audio)
Click on the link below to hear an audio recording of this article:
Juvenile HD is a form of Huntington’s disease that affects children and teenagers. Like the adult form of the disease, juvenile HD is hereditary in nature. Because of its hereditary character and early age of onset, a child with juvenile HD may also have a parent or other close family member who is affected by adult-onset HD at the same time. This tendency to affect multiple generations simultaneously places an even greater strain upon families who are affected by juvenile HD.
What is juvenile HD?^
Although juvenile HD and adult-onset HD both result from an altered form of the same gene (the Huntington gene), the symptoms of juvenile HD are very different from those of adult-onset HD. Individuals with juvenile HD often become stiff or rigid in their movements (instead of having chorea), and about one third of them have recurrent seizures. As with adult-onset HD, individual cases of juvenile HD vary greatly, and different children often have different symptoms. As a result, cases of HD are classified as the juvenile or adult form based upon age of onset, and not by symptom. Any case of HD where the onset occurs before the age of 20 is considered to be of the juvenile form, regardless of the symptoms present.
Although the number of CAG codon repeats in a particular segment of the Huntington gene does not accurately predict the age of onset, generally more repeats correspond to an earlier age of onset. This tendency is especially true in cases of juvenile HD, where most individuals have between 80 and 100 CAG repeats. The earlier the onset of juvenile HD, the faster it usually progresses. In general, progression of the disease is more rapid than in adult-onset HD. Often, death from juvenile HD occurs within 10 years of onset, as opposed to 10-25 years in adult-onset HD.
What causes the large CAG repeat numbers seen in juvenile HD cases?^
Children with juvenile HD usually have a larger number of CAG repeats in a particular segment of the Huntington gene than do individuals with adult-onset HD. In many cases, these children also have many more CAG repeats compared to the parents from whom they inherited the HD allele. The exact cause of repeat expansion is still unclear. At one point, it was thought that the DNA from the unaffected parent might somehow contribute to the development of juvenile HD. A non-HD allele from the unaffected parent could potentially “aggravate” the Huntington gene and somehow cause the large increase in repeat numbers characteristic of juvenile HD. Given that juvenile HD is so rare, if there existed an allele that aggravated the disease, it would necessarily be rare as well. A case study in the 1960’s showed a man with adult-onset HD who had affected children with two different women. In order for this to occur, both of the mothers must have had the rare “aggravating allele,” a highly improbable occurrence. This finding made it seem unlikely that DNA from the unaffected parent was contributing to the expanded repeats.
(For more information on DNA, click here.)
Along with this process comes the possibility that a mistake is made somewhere during the copying procedure. Such “mistakes” are very common during DNA replication. One such mistake might cause the number of codon repeats to increase. Since the formation of sperm involves millions of cell divisions more than the formation of eggs, the number of opportunities for triplet expansion during DNA replication is much larger in males than in females. Hence, it is possible that adult males are more likely to pass alleles with expanded repeat numbers to their children. We will call this the “paternal triplet expansion hypothesis.” This hypothesis could explain why in most cases (about 70-90% of them), individuals with juvenile HD have inherited the HD allele from their fathers rather than their mothers.
How are large repeat numbers related to the increased severity of juvenile HD?^
Individuals with early-onset HD usually have a number of CAG repeats in a particular segment of the Huntington gene that is much larger than the number of repeats seen in adult-onset HD. The largest number of CAG repeats seen thus far is around 250, but most individuals with juvenile HD have between 80 and 100 repeats.
(For another example of correlation and causation click here.)
Most individuals with juvenile HD experience an age of onset that is much younger than that of their affected parents. They also often face a much more rapid progression of the disease. This occurrence is described as genetic anticipation, where a disease increases in severity in successive generations, and a parent can produce a child with a more severe form of a disease. In the case of HD, the expanded section of triplet repeats provides a possible (though still unconfirmed) explanation for the pattern of anticipation seen in HD inheritance. As the number of repeats grows between generations, the severity of the disease increases, and individuals experience an earlier age of onset and a more rapid development of the disease.
How is juvenile HD inherited?^
Juvenile HD is caused by the same gene that causes adult-onset HD. The version of the Huntington allele causing early-onset HD usually has, however, a greater number of CAG repeats. Because the early-onset and late-onset forms depend upon the same gene, early-onset HD is inherited in the same manner as adult-onset HD. (To read about how the HD allele is inherited, click here.)
Due to the rapid progression of the disease, most individuals with juvenile HD do not survive to bear children of their own. For those who do, however, their children have the same 50% risk of inheriting the HD allele as the children of individuals with adult-onset HD. The number of CAG repeats in the Huntington gene of an individual with juvenile HD is normally very high (even compared to that of individuals with adult-onset HD). Since repeat numbers tend to increase rather than decrease in successive generations, it is likely that the child of such an individual will have a similar or larger number of repeats if he or she inherits the altered allele. Given the correlation between repeat number and age of onset (discussed in the previous section), it is very likely that the child will also develop juvenile HD. In short, the child of an individual with juvenile HD has a 50% chance of inheriting the HD allele. If the child does inherit the altered allele, he or she is very likely to develop juvenile HD.
What are the early signs of juvenile HD?^
Although by definition juvenile HD begins at an early age, most children are able to walk and talk at a normal age before symptoms start to appear. The signs of juvenile HD are often subtle and difficult to distinguish from the normal “growing pains” that children experience. A major sign of onset is a continuing decline in school performance. Other indications include subtle changes in handwriting, difficulty learning new things, and small problems with movement. Some common movement problems include slowness, clumsiness, rigidity, tremor, and muscular twitching, or myoclonus. Parents often notice that their children fall more often and are less coordinated than they used to be.
Every case of juvenile HD is unique, and it is possible that individuals experience different symptoms depending on the age of onset and exact number of CAG repeats. However, many parents of children with HD have said that the most noticeable aspect of onset is change. Parents might notice personality changes, new problems with coordination, behavioral changes, new speech difficulties, and changes of pace in learning. For example, a child who was once very good at sports has become clumsy in recent months, or a previously well-behaved student is suddenly causing trouble at school. A mother of two children with HD described her perception of the changes within her family members:
“Following the diagnosis of HD in the first child, I began, of course, to observe the other family members very closely – ever vigilant for signs of HD. Some things, such as moodiness, speech problems, or hyperactivity could have been interpreted as early symptoms of HD. It became apparent that the clue seemed not to be the action itself, but rather, whether or not those things had always been present or if they represented a definite change.”
What symptoms are common to both juvenile HD and adult-onset HD?^
Both the early- and adult-onset forms of HD are characterized by what is called dementia, a progressive loss of mental function. Many individuals also seem to undergo personality changes. Some changes, such as increased irritability and bad temper outbursts, are sometimes due to the difficulties of dealing with the disease rather than actual clinical symptoms. Often, people with HD experience frustration when realizing that they can no longer do things they once could. Sometimes, however, these personality changes are a more direct result of the disease. Such symptoms may be alleviated with medication.
For an explanation of these differences click here.
How are the symptoms of juvenile HD different from those of adult-onset HD?^
The most notable symptomatic distinction between the two forms of HD is that many individuals with juvenile HD do not experience the chorea that is so commonly associated with the adult-onset form. Instead of exhibiting the dance-like movements of chorea, affected children are often rigid and stiff. Generally, children with a younger age of onset are less likely to experience chorea. Chorea is more likely to be present in individuals who have an age of onset from 15-18 years. It seems that individuals with juvenile HD who have a later age of onset are more likely to experience symptoms that resemble those of adult-onset HD.
About 25-30% of individuals with early-onset HD also experience recurring seizures, a symptom that is uncommon in the adult-onset form. Seizures experienced by children with HD are usually generalized, meaning that they are caused by electrical discharges that affect both sides of the brain and often involve a loss of consciousness. However, some children also develop partial seizures, which involve discharges in just one part of the brain and may or may not involve a loss of consciousness.
The generalized seizures experienced by HD children are usually what are called tonic-clonic seizures. Generalized tonic-clonic seizures (or grand mal seizures) consist of both tonic and clonic phases. During the tonic phase the body is rigid, and often the child falls to the ground. The clonic phase follows the tonic phase and is usually associated with convulsive movements or rhythmic jerking motions. The child typically loses consciousness for a variable period of time.
Some children also develop myoclonic seizures, which involve sudden, brief jerking movements, or myoclonus. These seizures vary greatly in their severity and frequency. Myoclonus should not be mistaken for seizures – the term myoclonus refers to the jerking symptom itself, which can have many causes. It is only when myoclonus is caused by abnormal brain activity that it is properly called myoclonic seizure. Many children with HD experience myoclonus that is not related to seizures.
What parts of the brain are affected in juvenile HD?^

At autopsy, individuals who have died from juvenile HD show an even more widespread pattern of brain degeneration than that seen in adult-onset HD. As in the adult form, there is severe degeneration of the caudate and putamen. (See Figure D-4.) The caudate and the putamen are responsible for regulating voluntary movement, and it is thought that damage to these parts of the brain is responsible for many of the movement problems — especially the chorea — that individuals with HD experience. (See Figure E-1.)

A characteristic that is seen more often in the juvenile form than in the adult form is extreme gliosis of the globus pallidus (Figure E-1). Gliosis is excess growth of what are called spider cells (see Figure E-2) — cells that normally provide supporting and protective tissue for nerve cells. Some individuals with adult-onset HD experience rigidity (instead of chorea), and case studies of several of these individuals have also shown damage to the globus pallidus. Hence, it is thought that abnormality of the globus pallidus may be responsible for the rigidity seen in juvenile HD.
Analysis of juvenile HD brains shows damage to many areas, but the pattern of damage is not consistent between individuals. Loss of neurons in the Purkinje cells and granule cells of the cerebellum is often seen in the juvenile but not the adult form. Other areas of damage sometimes include the dentate nucleus, hippocampus, and neocortex. The dentate nucleus is responsible for rapid movements, and the hippocampus deals with the transfer of information from short-term to long-term memory. The neocortex constitutes about 85% of the brain’s total mass, and it is believed to be responsible for higher cognitive functions, such as language and memories. It is currently not known how damage to these areas of the brain manifests itself as symptoms in people with juvenile HD.
What treatments are recommended specifically for juvenile HD?^
Anticonvulsant drugs are usually prescribed to help prevent and control the seizures that occur in children with juvenile HD. Finding the right combination and amount of drugs is not an easy process, and often the optimal treatment varies over time and between individuals. In many cases, caregivers know the most about the child’s reactions to specific drugs, making it very important for the doctor and caregivers to communicate frequently about which drugs and doses are working and which are not.
Myoclonus and jerking motions are usually not treated unless they are very severe (for example, if they cause the child to fall frequently or reduce the child’s ability to take in food). Antimyoclonic drugs such as valproate are sometimes prescribed to treat myoclonic jerks.
Side effects of seizure drugs can include drooling, sleepiness, and a general sense of confusion. However, the most significant concern of seizure medications is their potential to aggravate other juvenile HD symptoms. Some drugs may cause increased swallowing problems, drowsiness, and coordination difficulties. Many children with HD also have a poor tolerance of anticonvulsant drugs. Generally, physicians attempt to minimize the seizures as much as they can without lowering the quality of life in other areas. Achieving this ideal balance often requires trying many different drugs and prescribing less than the maximum dosage of each particular drug. Although the children may still have occasional seizures, many parents consider this treatment more acceptable than the alternative: prescribing a higher dosage to eliminate seizures but worsening the child’s other symptoms.
Physical therapy is recommended to ease rigidity and to prevent degeneration (atrophy) of unused muscle. For some individuals, pool therapy especially helps to loosen tight muscles. Pool therapy involves exercises that are done while the individual is submerged in warm water. The warm temperature is soothing for muscles, and the buoyancy of the water makes motion require less effort, enabling patients to strengthen muscles gradually.
Drugs are sometimes prescribed to control other symptoms, such as rigidity and difficulty sleeping. Counseling and medication sometimes help with behavioral and psychological symptoms. Many times individuals with juvenile HD respond poorly to drugs that are commonly prescribed for adult-onset HD. Hence, with each new drug or dosage, the child should be monitored carefully for side effects, such as increased drowsiness or poorer performance in school. The most effective combination of treatments is different for every individual with juvenile HD, and this optimal care can be achieved only when the doctor and caregivers work together to discover what is best for the child.
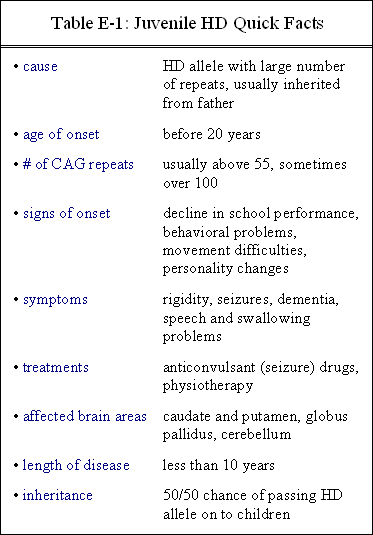
Table E-1 gives an abbreviated summary of juvenile HD.
For further reading^
- “Juvenile Huntington Disease,” Huntington Society of Canada. Online.
An excellent resource for juvenile HD; one of the few souces that focuses only on juvenile HD. - Byers, R. K., and J. A. Dodge (1967) “Huntington’s chorea in children. Report of four cases.” Neurology 17: 587-96.
A paper describing the symptoms, treatment, and diagnoses of four children with juvenile HD. - Byers, R. K., F. H. Gilles, and C. Fung (1973) “Huntington’s disease in children. Neuropathologic study of four cases.” Neurology 23: 561-9.
A very detailed paper that analyzes the brains of four individuals who died from juvenile HD and compares them to similar neuropathologic studies. - “Case 117 — Progressive Movement Disorder,” University of Pittsburgh School of Medicine, Department of Pathology. Online.
A case study of the diagnosis of a girl with juvenile HD.
A. Hsu, 2-25-02
Podcast: Play in new window | Download






