Cancer is caused by mutations in oncogenes and tumor suppressor genes. Mutations are changes in the DNA sequence that may alter gene function. Gain-of-function mutations can activate oncogenes, whereas loss-of-function mutations can inactivate tumor suppressor genes. Our laboratory studies the Myb oncogene family that is mutated in human cancers of blood cells (leukemia), brain, breast, and salivary gland. The proteins encoded by Myb genes bind to DNA and regulate the expression of other genes that control cell division, differentiation, and cell death. The Myb proteins interact with a highly conserved multi-protein complex called the MuvB core. The same complex also interacts with proteins of the Rb tumor suppressor family and the E2F transcription factor family. Work from our laboratory has shown that Myb acts in opposition to Rb-E2F to epigenetically regulate gene expression.
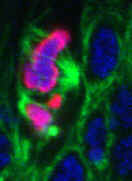
Left: Abnormal mitosis in a Drosophila Myb null mutant.
DNA (blue), condensed chromatin (magenta), and microtubules (green). [Manak, et al, PNAS, 2002; Manak, et al, Nature Cell Biology, 2007]
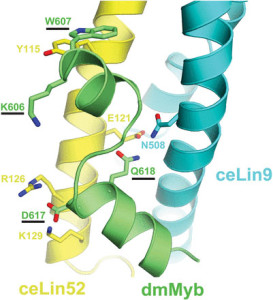
Right: Model of the MuvB-interacting domain of Drosophila melanogaster Myb protein binding to the Myb-interacting domains of C elegans Lin9 and Lin52. Amino acids that are required for binding in vitro and biological activity in vivo are underlined. The nematode C elegans lost its own animal-type Myb gene and protein over 500 million years ago. This model is based on a crystallographic structure of the homologous domains of human proteins: B-Myb, Lin9, and Lin52. The model is consistent with in vitro biochemistry and in vivo genetic studies [Guiley, et al, PNAS, 2018; Vorster, et al, Biology Open, 2020]