Coupling Isotachophoresis and Capillary Electrophoresis using Bidirectional Isotachophoresis
Supreet S. Bahga, Robert D. Chambers and Juan G. Santiago
Isotachophoresis (ITP) is an electrophoresis based technique, in which analytes simultaneously focus and separate between high effective mobility leading electrolyte (LE) ions and low effective mobility trailing electrolyte (TE) ions. The balance of electromigration and diffusion at the zone boundaries in ITP results in sharp moving boundaries, which can be described as ion concentration shock waves. Typically, ITP experiments are performed separately for focusing anions or cations in anionic and cationic ITP, respectively; however, anionic and cationic ITP can also be performed simultaneously in a single channel. The latter approach, called bidirectional ITP, is characterized by anionic and cationic ITP shock waves propagating in opposite directions.
Shock wave interaction in bidirectional ITP
In a first-of-its-kind study, we have demonstrated a bidirectional ITP process in which anionic and cationic ITP shocks approach each other and then interact. We have described how interaction of cationic and anionic ITP shocks in bidirectional ITP can lead to fundamental changes in focusing behavior of analytes. Figure 1 shows spatiotemporal plots obtained from numerical simulation and experimental visualization of shock interaction in bidirectional ITP.
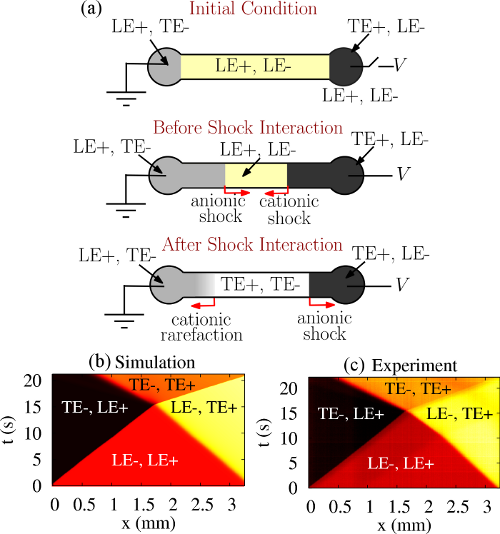
Figure 1: (a) Schematic of chip showing initial condition, shocks approaching, and after interaction (b) Numerical simulation and (c) experimental visualization of interacting anionic and cationic ITP shocks. The spatiotemporal plot shows the intensity of a fluorescent nonfocusing tracer (NFT) versus distance along the channel axis, x, and time, t. We set up two simultaneous ITP shock waves: an anionic shock between LE-/TE- ions and a cationic shock between LE+/TE+ (LE and TE stand for leading and trailing electrolytes). Prior to the shock interaction, LE-/TE- and LE+/TE+ shocks propagate toward the right and left, respectively. For the conditions shown here, after the shock interaction the rightward traveling LE-/TE- shock remains intact, while the leftward travelling LE+/TE+ interface is disrupted (and begins to disperse).
Coupling ITP and CE via shock wave interaction
Shock interaction in bidirectional ITP can be used to trigger fundamental modification of the electrophoretic conditions. For example, shock interaction can initiate changes in counterion species, concentration of co-ion species, local pH (e.g., changing effective mobility), and species zone order. We have demonstrated a bidirectional ITP process in which we use the interaction of anionic and cationic ITP shock waves to trigger a transformation from ITP preconcentration to electrophoretic separation (capillary electrophoresis, CE). We use anionic ITP to focus anionic sample species prior to shock interaction. We choose the electrolytes such that, the interaction of the counter-propagating anionic and cationic ITP shocks changes the local pH (and ionic strength) of the focused analyte zones. Under this new condition, the analytes no longer focus and begin to separate electrophoretically (see Figure 2).
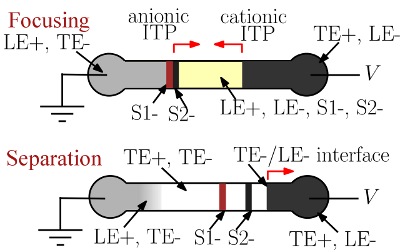
Figure 2: Schematic illustrating focusing and separation of analytes using bidirectional ITP. Buffers are chosen such that LE+ is a cation of a weak base with high mobility and TE+ is a cation of a strong base with low mobility. Furthermore, LE- is an anion of a strong acid with high mobility, and TE- is an anion of weak acid with low mobility. When voltage is applied, the anionic analytes focus between LE- and TE- and propagate rightwards. Simultaneously, a leftward-propagating cationic ITP shock forms between LE+ and TE+ zones. When the LE+/TE+ shock washes over the focused anionic analytes, it raises the pH of anionic ITP zones (as TE+ is a stronger base than LE+). This increase in pH of anionic ITP zones causes TE- ions to overtake the focused analytes. Consequently, the anionic analytes S1- and S2- cease focusing and commence electrophoretic separation.
Advantages:
- The method provides faster and much less dispersive transition from ITP preconcentration to electrophoretic separation compared with traditional (unidirectional) transient ITP.
- Because the transition from ITP to CE is automatic (due to shock interaction) the method eliminates the need for intermediate steps, such as buffer exchange and deactivation and reactivation of a power supply, that are usually required in transient ITP.
- Our technique can also be applied to conventional single-channel CE systems (e.g., using fused-silica capillaries) and eliminates the need for column-coupled channels for buffer replacement as in transient ITP.
Video 1: Simulation showing preconcentration and separation of two model analytes using bidirectional ITP.
Video 2: Experimental visualization of preconcentration and separation of 1 kb ds-DNA ladder using bidirectional ITP. The video shows DNA fragments focused in a thin ITP zone, prior to the shock interaction. After the shock interaction, the DNA fragments start separating as in capillary electrophoresis.
Reference
Bahga S.S., Chambers, R.D., Santiago, J.G., "Coupled Isotachophoretic Preconcentration and Electrophoretic Separation Using Bidirectional Isotachophoresis", Anal. Chem. 2011, 83, 6154-6162. (click here for pdf)
