[Menu] [Previous] [Next]
TUTORIAL: Principles of Tracer Modeling
PET Tracers: Models
Use the "Menu" button to jump to the Let's Play PET Main Menu or click on the Next (right arrowhead) and Previous (left arrowhead) buttons to proceed sequentially through the topics and tutorials. Or, you can return to the Department of Molecular and Medical Pharmacology's Home Page.Contents:
Topics:
Introduction
Tracer kinetic models in quantitative PET provide a mathematical framework for calculating the concentration of reactants and products, and the rate of biological processes. Compartmental models are the most common tracer kinetic models used in PET. These models represent simplifications of biological systems. The models are formulated by differential equations describing exchange between compartments. Compartmental models describe biochemical systems and therefore require extensive biochemical studies to define them, as well as simplifying approximations in their practical formulations. Once a model is formulated, a tracer kinetic assay can provide very sensitive and accurate measurements of the rates of the process.
Acetate Model
There does not yet exist a workable model of C-11 acetate for the study of oxidative metabolism with PET.
Ammonia Model
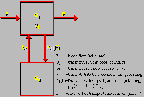
Click on image above to view full-size image.
Carbon Dioxide Model
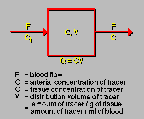
Click on image above to view full-size image.
Fluorine Ion Model
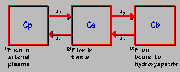
Shown above is the three compartment model of skeletal [18F]fluoride ion kinetics. Cp, Ce and Cb refer, respectively, to the plasma, extravascular and bound bone compartments. K1, K2, K3 and K4 are first order rate constants describing the potential directional exchanges between compartments. Physiologically, Ce represents [18F]fluoride ion in an extravascular space unbound to bone, while Cb refers to the activity bound to bone (either on the bone surface or fully incorporated into the hydroxyapatite crystal–fluoride ion exchanges with the hydroxyl groups in the hydroxyapatite crystal of bone). See hawkins, et al., 1992 for further details.
Fluorodeoxyglucose Model
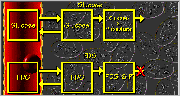
Click on image above to view full-size image.
The first three compartments in the metabolism of glucose are shown above. After its transfer to the tissue, glucose is phosphorylated into glucose-6-phosphate (G-6-P). The arrow projecting from the right of the third compartment symbolizes that G-6-P is further metabolized in the glycolytic pathway. (Dephosphorylation of G-6-P is not shown.)
[F-18]Fluoro-2-deoxy-glucose (FDG), an F-18-labeled analog of glucose, is used in PET to study glucose metabolism. After a bolus injection into the blood, FDG enters the tissue and is phosphorylated into FDG-6-phosphate (FDG-6-P). Because of the missing oxygen at the second carbon position, FDG-6-P can not be further metabolized in the glycolytic pathway.
During an FDG PET study (typically after a patient has fasted) the endogenous glucose level is relatively constant (i.e., at a steady state). Using a gray scale, with black indicating lowest concentration and white indicating highest concentration, the filled compartments in the glucose model reflect the steady-state nature of this system.
At the beginning of an FDG PET study, FDG is absent in both blood and tissue, as shown by the color black in the above FDG model. In such a study, FDG is typically injected as a bolus into the blood.
Following a bolus injection, FDG initially peaks in the blood and then accumulates in the tissue. The blood curve would come from well-counted blood samples taken during a PET study. The tissue-curve represents a corresponding plot of FDG concentration from analysis of a tissue region over a sequence of PET scans taken during a 45-minute study.
Fluorodopa Model
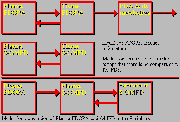
Click on image above to view full-size image.
Fluroethylspiperone Model
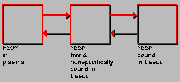
Click on image above to view full-size image.
Leucine Model
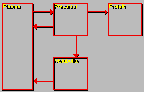
Click on image above to view full-size image.
Oxygen Model
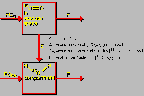
Click on image above to view full-size image.
Water Model
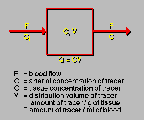
Click on image above to view full-size image.
[Menu] [Previous] [Next]