TAXONOMY |
| The virus family Parvoviridae contains 3 genera: the parvoviruses
, the dependoviruses, and the densoviruses (which only
infect insects). These viruses have no way of forcing an infected cell in replication, so they
must infect cells that are regularly dividing in order to be productive. The dependoviruses are
considered defective viruses because they require co-infection by either an adenovirus or
herpesvirus in order to replicate, whereas the parvoviruses do not, and are considered autonomous. |
|
GENOME |
| Interestingly enough, Parvoviridae is the only family of DNA viruses that infect humans that is
single stranded. The DNA strand packaged in the virion can be either negative or positive sense, though
in case of some strains, it is always negative. The linear genome is around
5.6 kb in length, and contains 5 genes, two non-structural (NS1 and 2) and either two (HPV B-19) or
three (AAV) structural (VP1, 2, and 3) proteins, depending on the virus. |
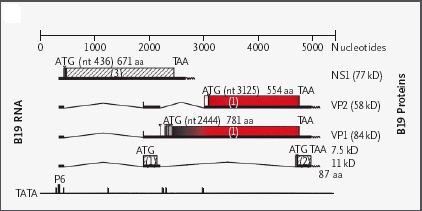
HPV B19 genome and mRNAs.[14] |
|
VIRION MORPHOLOGY |
| Parvoviruses are non-enveloped, icosahedral viruses with T=3 symmetry. These are the smallest of
the DNA viruses, with capsids measuring 25 nm in diameter. The name parvo comes from Latin for
"small." The VP2 protein makes up the majority of the capsid, with a small portion of VP1
extending out from the capsid as a receptor for host-cell binding. |
|
REPLICATION |
| Since the parvoviral genome is single-stranded DNA, it does not need any special polymerases for replication, and so
even naked viral nucleic acid is infections. The genome is self-priming because the ends are self-complementary,
forming hairpin turns at both termini. With the dependoviruses, both termini contain the same inverted repeats.
Replication occurs in the nucleus of the host cell, and involves double-stranded intermediates and the formation of
concatamers (multiple genomes strung together) which are then cleaved for packaging. The mechanism, however, for
this site-specific cleavage is unknown. For gene expression, parvoviruses use one or two promotors depending on
the species; if two, one is for regulatory proteins and the other is for structural proteins. The structural mRNAs
all share the same 3' end and are formed from differential splicingat a specific intron. Adeno-associated virus (AAV),
a dependovirus, also uses different reading frames with different startcodons in order to form the different mRNA
transcripts for VP2 and VP3. These viruses, like most DNA viruses, can also be integrated into the host genome
and cause latent and or persistent infections. |
|
|
|
 |
Panel A: Sample from a bone marrow biopsy. Panel B: Erythroid progenitors with nuclear inclusion
bodies, characteristic of parvovirus infection.
Panel C: HPV infected erythroid progenitors, stained using monoclonal
antibody against VP1 and VP2 capsid proteins.[3] |
|
|
| New Research Findings from 2003 and 2004: |
- In a study with 88 children with rheumatic disease and 40 adults with SLE, serum samples were tested for the presence of IgG antibodies
specific for both B19 proteins VP1,VP2 and NS1 and for antiphospholipid antibodies. They found that the antiiphospholipid antibodies
were preferentially found in the serum of children with arthritic symptoms where HPV B19 had established a persistent infection. With
the adults, they found a high correlation between antiphospholipid IgG and persistent B19 infection, concluding that B19 infection may be
directly responsible for the induction of antiphospholipid antibodies (autoantibodies) and the autoimmune reaction.[12]
- Using an adeno-associated virus vector containing angiostatin (a potent inhibitor of tumor growth in vivo), Lalani et al. were
able to show in mice that both B16F10 melanomas and Lewis lung carcinomas (human tumors) could be reduced as compared to control groups.
This has very important implications for anti-tumor gene therapy using AAV vectors.[5]
- In a similar study, an AAV vector containing cystic fibrosis transmembrane conductance regulator (CFTR) complementary DNA [tgAAVCF]
was administered to cystic fibrosis patients in aerosolized form. The study concluded that the CFTR-AAV vector improved pulmonary function
in every test subject who received the treatment. These are encouraging results for the use of AAV-mediated gene therapy for treatment of
genetic disorders.[6]
- The use of small interfering RNAs (siRNAs) for sequence-specific gene silencing is an important genetic research tool, especially since they
can be used in vivo. Tomar et al. showed the efficacy of using adeno-associated viral vectors for siRNA delivery into HeLA cells, both dividing
and nondividing cells. The experiment also showed the potential therapeutic value of siRNA delivery by successfully downregulating p53 and caspase 8
expression in the HeLa cells. This displays another possible route of cancer therapy using AAV-mediated siRNA delivery to silence specific genes.
[11]
- In a study with the autonomous parvovirus minute virus of mice (MVMi), Segovia et al. found that MVMi targets multipotent hematopoietic stem cells (HSCs) differentially
depending on what stage the progenitor cell was in during infection. This may shed some light on how and why some parvoviral infections lead to
persistent infections while others are unproductive. In the experiment, they also performed bone marrow transplants on MVMi-infected SCID mice
(severe-combined-immuno-deficiency, refers to cases where neither competent B nor T cells can be produced by the immune system due to a failure
to functionally produce the T cell receptor and immunoglobulins) and showed that the parvovirus was effective at suppressing the HSCs in vivo, allowing
the bone marrow graft to not be rejected. This has important implications as far as preventing graft-vs.-host disease in bone marrow transplantation
by infecting the graft HSC's with parvovirus before transplantation.[9]
|
|
| For a complete list of references, please click here. |
|
| Link to Pathogen Cards |
|
|





