RESEARCH
Research Tracks
Atrial fibrillation (AF)
Atrial fibrillation (AF) is the most common arrhythmia globally, with over 30 million estimated suffers, and a major cause of stroke, disability, and heart failure. Therapy for AF is currently suboptimal, with limitations of pharmacological (drug) therapy to either reduce ventricular rate (‘rate control’) 12 or attempt to suppress AF (‘rhythm control’) 13. Ablation therapy is thus increasingly used, and while successful in many patients the results of pulmonary vein isolation (PVI) at 1 year is 50-60% in paroxysmal14 and persistent15 AF in recent randomized trials with contemporary imaging and catheter systems16,17. Frustratingly, recent clinical trials have shown that common empirical ablation (linear lesions, complex fractionated atrial electrograms) may not improve results15.
Better mechanistic understanding is needed to improve ablation therapy for AF and to catalyze drug-discovery. Once AF is initiated by triggers from pulmonary veins 18, disorganized activity can be sustained by 2 hypothesized mechanisms. In one, AF is caused by a multitude of short-lived spiral waves (wavelets) with a limited spatial extent. In the multi-wavelet hypothesis19, disorganized activity generates new wavelets in a stochastic fashion. A related hypothesis is that transient focal activity cause AF20. This hypothesis does not require “special” tissue regions so that AF can occur in homogeneous or heterogeneous tissue. This does not explain clinical cases in which very localized ablation can treat AF 1-4,21, or why extensive ablation that should compartmentalize wavelets does not improve AF success rates15. An alternative mechanism is that “special” regions of the atria harbor sources in each patient, in the form of spiral waves or focal sources. These localized sources generate wavefronts that break down, known as fibrillatory conduction. This can explain the clinical observations listed above.
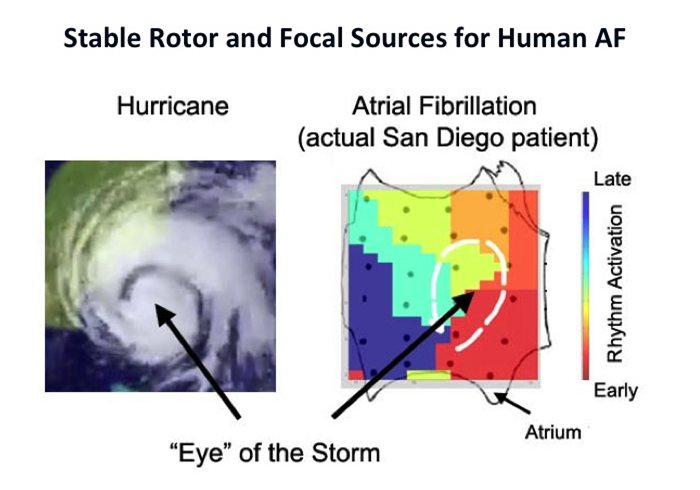
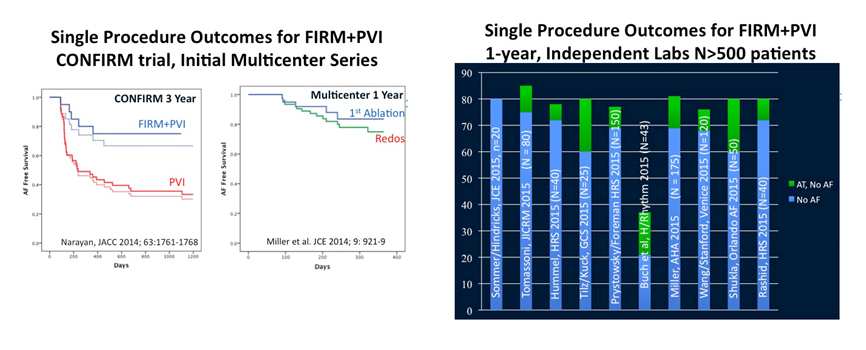
Our laboratory reported in 2011 that localized rotors and focal sources sustain human AF1,22, by developing Focal Impulse and Rotor mapping (FIRM) to map AF based upon physiological and technical considerations specific to AF23,24. AF sources meet classical criteria as drivers and, in a preliminary optical mapping study of AF in human atria, the rotors were identified simultaneously on both FIRM maps and optical maps10; further details are awaited. Ablation of human AF rotors has been shown to terminate AF in many patients, and to improve the success of ablation. Many of these results have been verified by groups using FIRM in the U.S. and Europe1-6, and by other groups using different methods7,8. These results have now also been validated by optical mapping of AF in human hearts9-11.
Ongoing research in the laboratory is further characterizing AF rotors, identifying the inter-relationship between AF-sustaining sources and more simple circuits such as atypical atrial flutter, and using these data to further improve the results of FIRM-guided ablation for AF. Two randomized trials are ongoing at Stanford. SUBSTRATE is randomizing patients with paroxysmal AF to PVI alone or FIRM-guided ablation alone (NCT 02169037). RECONFIRM is randomizing patients with persistent AF to PVI plus FIRM or PVI alone (akin to the STAR-AF2 trial) (NCT02456233). Dr. Paul Wang serves as PI for both trials.
Ventricular fibrillation (VF)
Ventricular fibrillation (VF) is a major cause of sudden death, that affects over 250,000 individuals in the U.S. alone. Clinical VF is a relatively unstudied rhythm, with limited interventional therapies. However, the ablation of VF is an emerging area in patients with multiple ICD shocks despite medications. Dr. Narayan has studied repolarization and conduction abnormalities in patients with VT/VF and controls since 1996, that led to our Laboratory reports on VF rotors in patients in 2013-14 and reports of clinical VF rotor ablation from 2013-present.

Detailed Electrophysiologic Characterization of Human Atria and Ventricles
Arrhythmias may require dynamic and fixed mechanisms 21. In AF, triggers such as ectopic beats 13, bursts of atrial flutter 22 or varying autonomic balance 23,24 may or may not dynamically initiate AF despite relatively fixed atrial architecture, surface curvature and fibrosis25.
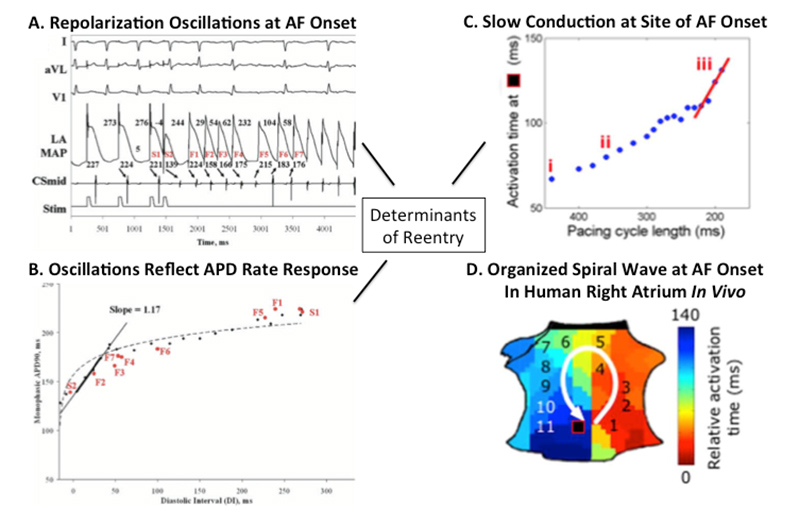
Since 2001, we have studied if the dynamics of repolarization and conduction may explain how triggers initiate clinical fibrillation, and then may be responsible for sustaining fibrillation. In the case of AF, figure 1A shows how a single ectopic beat produces dramatic oscillations of human left atrial action potential duration (APD)22,26,27, because of the fashion in which APD shortens with diastolic interval (DI, time between successive activations) or restitution (figure 1B) 28. Classically, a restitution slope of >1 enables an early beat (with short DI) to dramatically shorten APD and lengthen DI of the next beat, producing APD alternans which can be discordant in space and produce wavebreak. Atrial conduction velocity is also rate dependent. Figure 1C shows that triggers can dramatically slow human atrial conduction imminently before AF onset, producing spiral-wave re-entry at AF onset (figure 1D) 29. Although it is unclear how these dynamics interact with atrial anatomy30 or fibrosis31, a recent study showed that each of multiple distinct AF initiations (spiral wave reentry) occurred at spatially conserved sites for diverse triggers in both atria suggesting that functional mechanisms are spatially determined.
Novel Electrogram Analyses
Traditionally mapping of human AF has used classical rules 32 on electrograms that provide high temporal resolution and can be recorded at multiple sites. However, a number of potential pitfalls must be recognized when applying classical rules to interpret unipolar or bipolar electrograms given the spatiotemporal variability of AF.
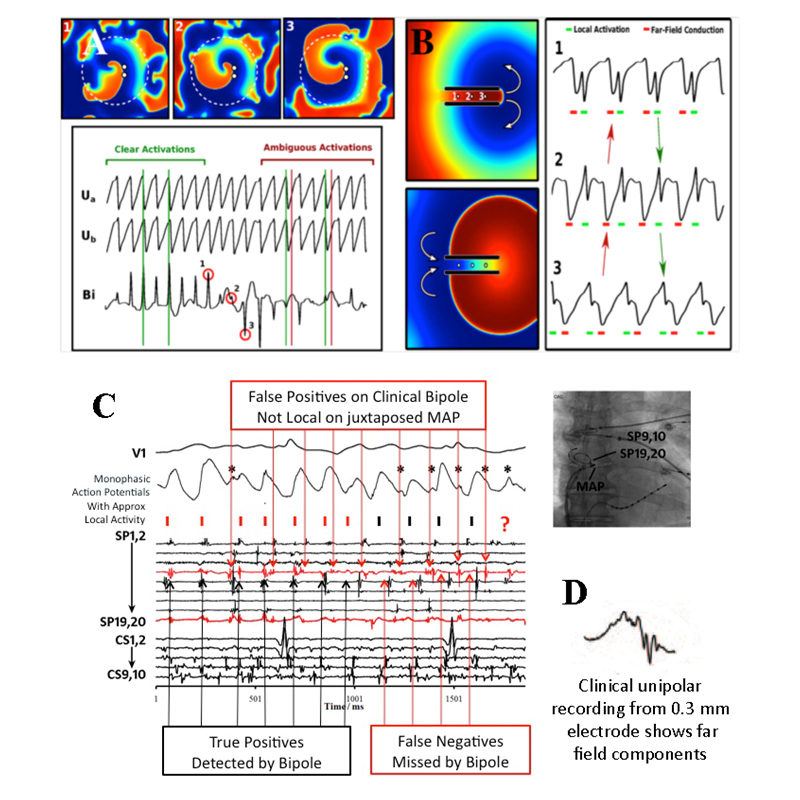
Recordings from bipolar electrodes, used in most clinical AF mapping studies, compare extracellular potential at 2 closely spaced poles. Bipolar signals depend on alignment of the electrode pair relative to an activation front which, if it reaches both poles simultaneously, will not be recorded and produce incorrect activation times. Figure 2A shows a computer simulation of a stable spiral surrounded by fibrillatory breakdown. The activation front arriving at the bipolar electrode (white dots in the coherent domain) is missed at point #2 because slight tip movement alters the activation direction relative to the bipole. This may result in ambiguous activation markings or electrograms that miss activation times. Clinically, we have demonstrated that bipolar signals in AF may completely miss local activation and also detect unrelated far-field activations on an opposed MAP catheter 33 (figure 2C).
Unipolar electrode recordings can also be misinterpreted in AF since, unlike optical mapping techniques, they integrate considerable far-field activation. Unipolar signals even from small 0.3 mm electrodes may reflect several far field components32 (fig. 2D) as shown in simulations34. This may occur even in simple rhythm – fig. 2B shows simulated macro-reentry through a narrow isthmus. Unipolar electrograms at isthmus locations 1, 2, and 3 display multiple deflections representing local (green) and far field (red) that make local activation time difficult to assign and can easily result in incorrect maps (red arrows). Electrograms may be more complex in a fibrotic milieu, that may further influence classical analysis.