
 |
 |
Phoenix System > Episomes > Tetracycline Regulated
Use of EBV-based Episomes for rapid, helper-free, stable recombinant retrovirus production.
The nuclear replication and retention functions of the Epstein Barr virus have been used to maintain retroviral vectors episomally within human-based retroviral packaging cell lines. These hybrid EBV/retroviral vectors are capable of producing helper-free recombinant retrovirus as soon as 48 hours, and for at least 30 days, after transfection into 293T-based ecotropic and/or amphotropic retroviral packaging cells. Viral titers greater than 10^7 CFU/ml were obtained after puromycin selection of transfected retrovirus packaging cell lines. This episomal approach to retroviral production circumvents limitations inherent in transient and chromosomally-stable retroviral producer systems thereby affording reproducibly rapid, large-scale, high-titer retrovirus production.
We have created Epstein-Barr virus-based retroviral vectors that take up stable residence as episomes within human retrovirus producer cells at an efficiency close to their transient transfection fre quency. In human 293 cells the rate of stable establishment of EBV-based episomes can reach 25% of the starting cell population after CaPO4-mediated transfection. To establish as stable episomes, it is required that the Epstein Barr virus Nuclear Antigen (EBNA) binds to the EBV origin of replication and nuclear retention sequences. The episome is thereby maintained at 5 to 20 copies per cell for up to two to three months, given a puromycin-resistance gene resident and selected for on the plasmid. The prototype retrovirus vectors use lacZ as the marker for rapid, accurate determination of titre. The vector incorporates a number of important features. First, a more efficient retroviral LTR has been designed into the vector, based upon the MFG retroviral backbone of Mulligan and colleagues. Second, the vector has been designed to accept retroviral backbones from a number of different systems, thus facilitating the movement of previously designed retroviruses based on the old systems into this more efficient version. Third, as is common in such cloning vehicles, a suitable polylinker region has been incorporated. In tests of this new vector we have determined that it will be possible to readily generate titres as high as 10^7. With further optimization we expect to reach titres in the 108 range, as we observed for the puro vector. Note that these titres are achieved within 5 to ten days after initiation of the culture and are higher than other reported systems. With the system we have observed that certain promoter/gene combinations provide up to 10^8 infectious virions per ml- normal levels are between 10^6 to 10^8. We expect it will be possible to transfer the high titre capacity of some vectors to other vector configurations. This new ap proach to generating recombinant retrovirus takes the best qualities of the transient system, which is limited to the ready production of up to 20 ml of high titre supernatent per virus, and weds it to the advantages of long-term outgrowth of retroviral producer lines. Within one week of initiation of the culture it is possible to obtain a few hundred ml of viral supernatent due to the rapid establishment of episomes in the cells. One can rapidly produce several liters (if necessary) of high titre viral supernatent within a few weeks of construction of a given recombinant retrovirus.
EBV Episomes for Rapid, Stable Retrovirus Production for Gene Therapy.
Todd Kinsella and Garry P. Nolan
This outlines our work to date to develop our rapid transient retrovirus production systems to allow for large-scale generation of biomedically relevant levels of high titre recombinant retrovirus for use in gene therapy and acdemic applications. We have created a novel transduction system that builds upon my previous work developing rapid transient high-titre retroviral production systems. The new approach will allow for very rapid stable, high titre production of retroviruses with ecotropic, amphotropic, and polytropic host ranges. Our proposed system relies on the creation of vectors and cell lines that allow rapid establishment of retroviral producer DNA as EBV-based episomes within 293 cell human producer systems. Thus, large recombinant producer cell populations-- and bulk retrovirus for concentration-- can be readily generated from small seed populations using this approach.
Epstein-Barr virus-based vectors take up stable residence as episomes within human cells at an efficiency close to their transient transfection frequency (22-28). In human 293 cells the rate of stable establishment of EBV-based episomes can reach 25% of the start ing cell population after CaPO4-mediated transfection. To establish as stable episomes, it is required that the Epstein Barr virus Nuclear Antigen (EBNA) binds to the EBV origin of replication and nuclear retention sequences. The episome is thereby maintained at 5 to 20 copies per cell for up to two to three months, as long as some drug marker is resident and selected for on the plasmid. After three months of constant selection there is evidence that the EBV vector can integrate into chromosomal DNA by non-homologous recombination (22, 23) and a non-episome cell population is selected. Within cells episomes under no selection are lost at a low, but constant, rate (22-28). It has been shown that high level expression of episomally based genes is readily accomplished (22, 27, 28).
We have taken advantage of the high transient transfectability of human 293 cells and the high rate of stable establishment of episomal resi dence afforded by EBV-based vectors in human cells. Together, the system allows for rapid establishment of stable cell lines that can be grown for large-volume production of recombinant retroviral virions. For instance, transfection of 30 ug of retroviral plasmid into 10^7 293-based retroviral producer cells should yield a transfected population of 2.5x10^6 producer cells (at 25% transfection efficiency). After 6 days selection for the episome (see below), providing a cell doubling time of 16 hours (unpublished), we expect a population of 1.28x10^9 cells. 293 cells can be adapted to spinner culture (22-28), thus allowing for very high density cell growth and retroviral production.
We have already shown that the basic approach we propose here can produce retroviral titres as great as 10^8 per ml in both ecotropic and amphotropic backgrounds (Todd Kinsella and G. Nolan, Human Gene Therapy). This is at least an order of magnitude higher than standard retrovirus production systems. In addition, these titres can be achieved within one week (other procedures require up to three months to achieve titres in the range of 10^6). To demonstrate this proof of principle, we constructed the EBV-based retroviral episome plasmid as shown in Figure 2. The vector has as selectable marker the puromycin resistance gene expressed within the retroviral backbone of a pBabe vector (9). At a unique Not I site in the vector we have placed the EBNA and EBV origin of replication to provide a means for episomal maintenance. Selection for the plasmid is provided by puromycin (this drug has been chosen due to its low cost relative to other selectable markers such as G418/neo and Hygromycin/hyg).
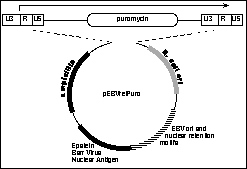 |
Puromycin-expressing retrovirus vector with EBV-ori and EBNA sequences for episomal expression. The retrovirus backbone is shown at the top of the figure with the 5' and 3' LTR regions flanking the expressed puromycin resistance gene. The EBV origin and Epstein-Barr Nuclear Antigen (EBNA) that establish and maintain episomal residence within human cells are as marked. |
We transfected this construct, pEBVretroPuro, into 293T-based retroviral helper cells to test its short term and long term ability to produce high titre virus. Two days after transfection, virus was collected from the transfected cells (day 2 virus, see Table below) and frozen. The transfected cells were split into puromycin selection conditions. Thereafter, virus was collected on day 6, 10, 14, 18, and 30. The results demonstrate that a control plasmid containing no EBV ori or EBNA sequence, virus was produced transiently, and that the majority of the cells in the producer population died upon selection. In contrast, cells transfected with the pEBVretroPuro survived and produced extremely high titre virus for up to 30 days post transfection (see published manuscript).
Since most retroviral vectors do not provide EBNA/EBV ori, we designed a new vector as shown in the next Figure. This vector places the puromycin selectable marker outside the retroviral backbone. In addition, the vector contains only the EBV ori sequences and will be used in a system in which EBNA is provided from a chromosomally integrated construct. In the future, this will minimize the possibility that EBNA sequences will non-homologously enter the retroviral backbone during transient transfection and episomal maintenance period, enhancing the systems' value in gene therapy experimentation.
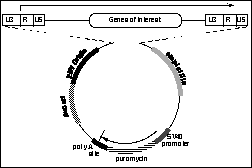 |
Design of vector for accepting a variety of retroviral backbones to allow episomal maintainance. The vector is shown with the retrovirus at the top of the figure inserted into the vector which contains a puromycin resistance gene expressed from SV40 origin. An EBV origin is depicted. EBNA is proposed to be expressed from a chromosomal, integrated state. |
The first of these vectors used lacZ as the marker for rapid, accurate determination of titre. The vector incorporates a number of important features. First, a more efficient retroviral LTR has been designed into the vector, based upon the MFG retroviral backbone of Mulligan and colleagues. Second, the vector has been designed to accept retroviral backbones from a number of different systems, thus facilitating the movement of previously designed retroviruses based on the old systems into this more efficient version. Third, as is common in such cloning vehicles, a suitable polylinker region has been incorporated.
It is possible to readily generate titres as high as 10^7. With further optimization we expect to reach titres in the 10^8 range, as we observed for the puro vector. Note that these titres are achieved within 5 to ten days after initiation of the culture and are higher than any reported system. We expect that this new approach to generating recombinant retrovirus will take the best qualities of the transient system, which is limited to the ready production of up to 20 ml of high titre supernatent per virus and wed it to the ad vantages of long-term outgrowth of retroviral producer lines. Thus, the system is capable of rapidly producing several liters (if necessary) of high titre viral supernatent within a few weeks of construction of a given recombinant retrovirus.
Download Material Transfer Agreements for Phoenix lines.
Home Page |
Interests | Members
|
MTA Forms | Plasmid
Maps | Retroviral Systems
| Genetic Screens | Library
Systems | Protocols
| Tutorials |
Publications | Contact
| Virus Chat