
 |
 |
Phoenix System > Episomes > Tetracycline Regulated
Tetracycline-regulated retroviruses.
See the manuscript by Hoffman, Nolan, and Blau for more information. Also see review by Schatz and colleagues in same issue of PNAS on Tet-regulated systems.
Requests for this and related reagents should be directed to the Blau Laboratory (hblau@cmgm.stanford.edu) for permission to obtain the vector and its receipt.
Rapid retroviral delivery of tetracycline-inducible genes in a single autoregulatory cassette. Hofmann A; Nolan GP; Blau HM.
Proceedings of the National Academy of Sciences of the United States of America, 1996, 93:5185-90.
Bujard and colleagues recently described a genetic control system based on the tetracycline repressor from E. Coli (82-84). The approach allows tight regulation of expression when genes are placed under the regulatory control of a TetR-VP16 fusion protein. The TetR-VP16 protein fuses the VP16 transcriptional activa tion domain to tetR. Thus, in mammalian cells, when TetR-VP16 binds the TetR-specific 19 base pair DNA motif upstream of a minimal promoter, that promoter will be activated. In the presence of tetracycline (1 uM doxycycline) the promoter is shut off.
A retroviral vector has now been constructed to allow inducible expression of retroviral inserts after integration of the vector in target cells; importantly, the en tire system is contained within the single retrovirus. Tet-inducible retroviruses have been designed incorpo rating the Self-Inactivating (SIN) feature of a 3' LTR enhancer/promoter retroviral deletion mutant. The enhancer/promoter region in the 3' LTR of this vector has been deleted from the first Pvu II site to the Sac I site, thus deactivating the LTR of the next generation. The U3 region of the 3' LTR is used during retroviral reverse transcription for creation of the next generation's 5' LTR enhancer promoter region. In essence then, removal of the 3' LTR promoter and enhancer elements from a recombinant retrovirus leads to the production of retroviruses that are transcriptionally defective, after integration in the target cell. However, internal incor poration of a promoter conferring constitutive, stage-specific, or inducible expression can allow for non-retro viral control over insert expression. This self-inactivating feature of retroviruses has been broadly used and is a well-represented technology in the published literature.

We have used this system to show that we can obtain efficient, reversible induction of gene expression by tetracycline-dependent regulation in a single retrovirus construct. The vector contains both the regulatory unit controlled by Tet binding (the Tet-Op minimal promoter of Bujard and regulator, the TetR-VP16 DNA-binding transcriptional regulator. Expression in this vector in cells is virtually undetectable in the presence of tetracycline or other active analogs (Figure 2). However, in the absence of Tet, expression is turned on to maximum within 48 hours after induction. Importantly, as assayed by flow cytometric analysis of lacZ reporter gene expression, we observed uniform increased expression of the whole population of cells that harbor the inducible retrovirus, indicating that expression is regulated uniformly within the infected cell population (Figure 2, right hand panel). In a second, related system described by Bujard the Tet DNA-binding domain was mutated such that it bound DNA in the presence of Tet, and was removed in the absence of Tet.
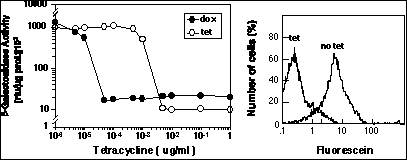
Results with retrovirus vectors express ing Tet-Op control ele-ments. Left hand graph shows dose-response curve of shutdown of gene ex pression with two different analogs of tetracycline. Shutdown rate after achieving effective dose range is 24-48 hours and dependent upon the half-life of the protein/mRNA being expressed. This effect is completely reversible, as shown in the right hand panel. The right hand FACS figure shows two time points for expression in the population after tetracycline withdrawal. Note that the population moves largely as a uniform peak. The two time points are 0 hours (Tet) and 48 hours after Tet withdrawal (no Tet). Bulk measurements of induction over background in these populations show nearly two orders of magnitude induction.
To obtain more information about this vector, please see the PNAS paper.
Download Material Transfer Agreements for Phoenix lines.
Home Page |
Interests | Members
|
MTA Forms | Plasmid
Maps | Retroviral Systems
| Genetic Screens | Library
Systems | Protocols
| Tutorials |
Publications | Contact
| Virus Chat