Supplementary Links and Notes for Stanford CS379C Spring 2012
Introduction to Neuroscience — Bear, Connors and Paradiso [4] — “Neuroscience: Exploring the Brain”
This is an excellent college-level introduction to the field of neuroscience. As an introductory text, it does a great job of covering this extensive field and the writing and illustrations nicely complement one another. There is also a good deal on-line supplementary material for the independent learner. See for example their graphical rendering of the Peripheral Nervous System: Somatic Sensory System — Afferent Processes — see Page 392 in [4], and the Autonomic Nervous System: Sympathetic and Parasympathetic Systems — Opposing — Efferent Processes — see Page 493 in [4] and Figure 7 below. I have used selected chapters as additional reading for my primer on the basics of neurons, neuronal signalling, mechanisms for learning and the human visual system. Lectures 1–4 in the class I teach at Stanford CS379C cover this material and the slides are available on the class web site under handouts. If you want an even easier introduction or just need a quick review, you might check out some of video lessons available from the Khan Academy under biology.
Neuroscience and Developmental Biology — Genes and Neurons Inextricably Linked — see here for a paper illustrating this connection.
If you become interested in neuroscience you’ll soon run into discussions concerned with genes and the cellular machinery that depends on genes. The claim made by Sapolsky that we consider in this study hinges on an analysis of the genomes of human and non-human primates. In addition to the role of genetics in prenatal development — Sapolsky refers to prenatal neurogenesis in stating his claim, genetics plays an important role in almost every aspect of how our brains work. In my presentation, I’ll refer to the processes of transcription and translation which together comprise gene expression. Inside the nucleus, single-strand template DNA is transcribed to make messenger RNA (mRNA) which is small enough to exit the nucleus. The mRNA is then translated by ribosomes residing outside of the nucleus to build proteins from amino acids — see Figure 10 below. There are a number of good — albeit highly simplified and overly dramatized — educational videos on YouTube which animate this process. The process of regulation occurs at several steps during gene expression: during transcription, RNA processing, RNA transport and during translation.
Michael Gazzaniga [25] — “The Cognitive Neurosciences”
Gazzinga studied under Roger Sperry and this book [25] is a worthy addition to your reference library. He is also a very effective public speaker and author of several excellent popular books on neuroscience — see this review of his latest offering in the Wall Street Journal [69]. There is a wonderful trove of Gazzaniga’s public speaking in his six Gifford Lectures which were given at Edinburgh University in 2009, and they are available directly from Edinburgh here or from the University’s YouTube channel. Lecture 2 “Distributed Networks” and Lecture 3 “The Interpreter” provide a good introduction to the experiments on so-called split-brain patients where the corpus callosum is cut in patients with severe epileptic seizures in an attempt to prevent seizures from spreading between the two hemispheres. I mention this series in particular because in these lecture Gazzaniga offers a different take on Robert Sapolsky’s conjecture — see here. Lecture 4 “Free Yet Determined and Constrained” covers the famous Libet experiments and other work relating to conscious decision making and various definitions of “Free Will.” Lecture 5 “The Social Brain” provides an overview of the Social Brain Hypothesis — see the work of David Premack, the consequences of a dramatic increase in clique size between 10,000 and 8,000 BC, and the discovery of motor neurons — see the work of Giacomo Rizzolati. Finally, Lecture 6 “We Are the Law” examines the role of neuroscience in the courts and, more generally, how neuroscience might play a role in shaping our understanding of culpability and retribution. In “Human” [24] Gazzaniga writes “We are social to the core. [...] Our big brains are there primarily to deal with social matters, not to see, to feel, or to cogitate about the second law of thermodynamics.”
Multitude of Mechanisms — “If there are a ten ways of doing something, you can bet nature has tried them all.”
The mechanisms for inter-cellular signalling in the brain and elsewhere are myriad. There are multiple genetic, chemical and electrical mechanisms or pathways. In the brain, we know of dozens of neurotransmitters, there as many or more genetic pathways and the propagation of action potentials along axonal processes is just one of several methods of signalling relying on electrical charge. But before you characterize this diversity of mechanisms as messy, think of the myriad means of communication and signalling in use today in modern society. We still have use for blinking lights and semaphore flags as backup emergency signalling. So-called “snail” mail is still alive and well. There is hardly any part of the accessible electromagnetic spectrum that we haven’t made use of for transmitting data. In addition to television and radio, we use lasers to communicate either short range through the atmosphere or longer range via fiber optic cables. Local wireless and mobile phone networks use increasing ranges of the spectrum and microwaves are used for point-to-point transfers. Conventional telephony using twisted pairs and coaxial cable are still commonly used by some telephone companies and for local area networks. For much the same reasons that nature isn’t likely to abandon an existing technology that works, commercial communication networks have a hard time cutting off customers or abandoning reliable technology and infrastructure. Natural selection can’t simply jettison an old design and start from scratch, and so it builds on or adapts existing mechanisms while sustaining robust germ lines.
Paul Maclean (1913-2007) — Ernst Haeckle (1834-1919) — Discredited Triune and Recapitulation Theories
Maclean’s Triune Brain Theory is comprised of a reptilian component (the basal ganglia), a mammalian component (the limbic system), and a primate component (the neocortex). Haeckle’s Recapitulation Theory was drilled into generations of biologists with the pithy phrase “Ontogeny recapitulates phylogeny” — see here for more discussion.
Karl Popper (1902-1994) — In science you can “let your hypothesis die in your stead” — see here.
George E. P. Box — “Essentially, all theories are wrong, but some are useful.” — see here.
Max Planck (1858-1947) is quoted as saying “Eine neue wissenschaftliche Wahrheit pflegt sich nicht in der Weise durchzusetzen, daβ ihre Gegner überzeugt werden und sich als belehrt erklären, sondern vielmehr dadurch, daβ ihre Gegner allmählich aussterben und daβ die heranwachsende Generation von vornherein mit der Wahrheit vertraut gemacht ist.” which is roughly translated as “A scientific truth (Wahrheit) does not triumph by convincing its opponents (Gegner) and making them see the light, but rather because its opponents eventually die and a new generation grows up that is familiar with it.” There are numerous paraphrases including “Die Wahrheit triumphiert nie, ihre Gegner sterben nur aus” translated as “Truth never triumphs — its opponents just die out.” and, my favorite one, this clever and succinct version which I heard from John Tooby of evolutionary psychology fame: “Science advances one funeral at a time.”
Philip Lieberman [40] — “Human Language and our Reptilian Brain” — Basal Ganglia — see here.
Primate Neurons — Spiking Neurons — Hundreds of Variations — Three Basic Classes — see here.
Afferent or sensory neurons, efferent or motor neurons, and interneurons which connect neurons to other neurons within the same region of the brain or spinal cord.
Contrast with C. elegans — Nematode — 302 Neurons — Specialized Analog Computers — see [11] and here.
Cerebral Cortex — Regular Structure — Single Algorithm Hypothesis — see here, here and Figure 1.
This was roughly the consensus of the three speakers — James DiCarlo, Geoffrey Hinton, and Michael Lewicki — at the symposium entitled “Learning and high-level vision: from single neurons to computational theory”, held on March 2, 2010 in Clark Auditorium at Stanford University. The basic premise goes back more than three decades. In 1978, Vernon Mountcastle [46] proposed that “all parts of the neocortex operate based on a common principle, with the cortical column being the unit of computation” — see here for more on Mountcastle’s contributions to neuroscience. Saxe et al [62] suggest that existing algorithms — including independent components analysis, sparse autoencoder neural networks, restricted Boltzmann machines and sparse coding, all of which yield similar results with no single algorithm obviously superior — might provide “phenomenological models of receptive-field plasticity during an organism’s lifetime” and a constructive realization of Mountcastle’s theory that a “qualitatively similar learning algorithm acts throughout the primary sensor cortices.” In a similar vein, Emanuel Todorov [71] argues that sensory input is handled by a form of Bayesian inference that combines bottom-up and top-down components and that additional, intermediate levels of representations can be stacked to form a hierarchical system much as was described in earlier work by Lee and Mumford [37]. He suggests that “different levels of generative model can be instantiated in different brain areas, and as long as the communication within and between areas corresponds to Bayesian inference, the entire distributed system will perform a single computation, using perhaps a single algorithm [...] which operates in parallel on multiple representations.” Relative to this hypothetical single algorithm, Todorov believes that the same basic idea can be applied to the motor system using the notion of a motor synergy as an analogy for sensory features.
Charles Darwin [13] (1809-1882) — “The Descent of Man” (1871)
”The difference in mind between man and the higher animals, great as it is, is one of degree not of kind.”
Robert Sapolsky — “Are Humans Just Another Primate?” — Academy of Science Lecture — one hour and fifteen minutes into the video link provided here.
The biggest difference between chimp and human brains is that we have three times as many neurons. The genes responsible for this difference govern the number of rounds of cell division during fetal brain development. “Take a chimp brain fetally, and let it go for two or three more rounds of cell division, and you get a human brain instead, and out pops symphonies and ideologies and hopscotch and everything else. What that tells is you is that with enough quantity you invent quality.” — Stanford Professor Robert Sapolsky in answering a question following his talk entitled “Are Humans Just Another Primate?” at the California Academy of Sciences in February 2011. According to Gordon Shepherd in The Synaptic Organization of the Brain [Oxford University Press, 1998, Page 6] we have a total 10 billion neurons in the cerebral cortex. However, Christof Koch lists the total number of neurons in the cerebral cortex at 20 billion in Biophysics of Computation: Information Processing in Single Neurons, [Oxford University Press, 1999, Page 87]. In humans, the cerebral cortex accounts for 77% of the brain by volume compared with 31% in rats [Trends in Neurosciences, 18:471-474, 1995]. The total volume of the cerebral cortex in humans is distributed as follows: frontal lobe = 41%; temporal lobe = 22%; parietal lobe = 19%; occipital lobe = 18%. [Caviness et al. Cerebral Cortex, 8:372-384, 1998] — see here.
Dean Falk [18] — Size Alone Can’t Account ... — see here.
“Despite the enormous energy that palaeoneurologists have devoted to studying primate brain size evolution, there remains a conviction that size alone is not enough to account for the observed diversity in primate behavior, and that circuitry, neurochemistry, and subsystems (modules) must have become reorganized within brains to accommodate evolving behavioral repertoires. Preuss [59], in fact, goes so far as to suggest that ‘the cortex is a veritable hotbed of evolutionary reorganization’. Although reorganization was undoubtedly important, deciphering the details of internal brain evolution is much more difficult than studying the gross phenomenon of brain size. Nevertheless, information yielded by both direct and indirect methods sheds some light on at least the broad aspects of neurological reorganization that occurred during primate evolution.”
Andrew Maas asked “Dolphin brains are as big as human brains; why aren’t dolphins as smart?”
I don’t know that much about dolphin brains or other Cetaceans except for a few experiments concerning dolphin decision-making abilities that I recall from Gazzaniga [25]. I don’t know that much more now having made the requisite check of Wikipedia. What I have learned about absolute and proportional (allometric) brain size comparisons, I’ve learned from reading [24, 25] and the work of Todd Preuss. I have summarized some of Gazzaniga’s observations here, but mention a couple of points that might provide a more satisfactory answer to your question.
Absolute brain volume has actually decreased by about 10% over the evolutionary history of H. sapiens and the proportion of white and gray matter in humans is quite different from other mammals and non-human primates in particular, with a marked increase in white matter — myelinated axonal processes — in the case of humans. As Preuss [59] and others have pointed out, bigger brains result in longer distances between functional areas. The neurons in all mammals are very similar and have the same capacity to support axonal and dendritic processes. H. sapiens seem to have evolved higher transmission speeds between functional areas and there is a pronounced increase in the rate of myelination starting during adolescent development and continuing on well into early adulthood.
Perhaps larger mammals with their, in many cases, larger but simpler and likely computationally-less-efficient brains are able to justify the metabolic demands in terms of some social-related advantages. Perhaps they can think deeply but much slower than humans, but I doubt it. My guess is natural selection favors larger brains for many reasons but that in general larger is not better. As one might predict given the focus of much of his research, Gazzaniga thinks the most compelling evidence comes from split-brain experiments; he points out in [24] that “[f]ollowing surgery the left brain can no longer communicate with the right [...] [I]n effect, a 1340-gram interconnected brain has become a 670-gram brain. What happens to intelligence? Well, not much. [...] The left hemisphere is the smart half of the brain. [...] [T]he left hemisphere remains as cognitively adept as it was before it was disconnected from the right brain leaving its 670-grams in the dust.”
Evidence for the Single-Algorithm Hypothesis — Rewiring the Ferret Cortex
There is an often mentioned — and as often misquoted — paper on “rewiring” ferret cortices to study functional plasticity in the auditory cortex [77]. I found the paper intriguing but inadequate for my purposes as it failed to characterize how the rewired A1 compared to V1 for which it was being substituted. The following book chapter provided the necessary literature survey that filled in many of the missing pieces:
“The formation of cortical layers and the arealization of cortex seem to be primarily influenced by intrinsic factors such as differential expression of gene families and molecular gradients in the developing cortical plate, before the arrival of axons from the thalamus. [...] However extrinsic factors, such as the amount and pattern of electrical activity in input pathways, also contribute to cortical development. Electrical activity generated within the developing brain may be sufficient for the establishment of thalamocortical connections, as suggested by the existence of retinal waves of spontaneous activity and the presence of ocular dominance columns before eye opening. [...] Visual activity, which has a very different spatial and temporal pattern than auditory activity, leads to visual responses in “rewired” A1 that resemble responses in primary visual cortex (V1).[...] [N]eurons in rewired A1 develop visual response features such as orientation selectivity, direction-selectivity, and an orderly retinotopic map. [...] Although the organization of visual information and connectivity of rewired A1 is similar to V1, there are several notable differences. For instance, the orientation domains in rewired A1 are larger and less orderly than in V1. [...] These differences may also reflect underlying structural constraints imposed by A1 that cannot be modified by experience (i.e. the structure of A1 cortical layers).” — excerpt from the chapter entitled “Rewiring Cortex: Functional Plasticity of the Auditory Cortex During Development” in the collection Plasticity of the central auditory system and processing of complex acoustic signals [50].
Jacques Lucien Monod (1910-1976) — 1965 Nobel Prize with Francois Jacob — see here.
“What is true for E. coli is true for the elephant.”
Jay Giedd [27, 70] — Adolescent Brain Development — see here and here.
Mark H. Johnson [31] — Developmental Cognitive Neuroscience — Interactive Specialization
According to which “development is not a unidirectional maturational process, but rather a set of complex, dynamic and back-propagated interactions between genetics, brain, body and environment. Development is not a simple question of a brain being built according to a pre-specified genetic blueprint — rather, the components of the brain are interacting with each other constantly — even prenatally, when patterns of spontaneous firing of cells in the eyes (before they have opened) transmit signals that appear to help develop the layered structure of the lateral geniculate nucleus .
The hypothesis has attracted increasing attention in recent years as a number of neuroimaging studies on younger children have provided data that appears to fit specific predictions made by Johnson’s model.” — see here for source.
Body Plans [43] — Homeoboxes — HOX genes — Mouse and Fruit Fly — see here and here.
Number of Segments [54] — Cyclopia — The eyeless gene [6] — Number of Digits — Sonic Hedgehog Gene [10]. In biology, morphology is a branch of bioscience dealing with the study of the form and structure of organisms and their specific structural features — see here. Thomas Jessell is a Professor at Columbia University and co-author of one the most cited textbooks on neuroscience — Kandel, Schwartz and Jessell [32] — and in this two-part video he provides a quick introduction to early embryonic development including cell division and differentiation and how the Sonic Hedgehog gene uses molecular gradients to guide cell growth and migration: here is Part I and Part II. If you want to delve deeper, you can watch the longer — one hour — version which was part of the Howard Hughes Medical Institute 2008 Holiday Lectures on Science here or you might read Chapter 54 of [32] which Jessell co-wrote with Joshua Sanes.
Genetic Basis for Neural Development — see here.
The material I found on the web was not as specific as I had hoped for — or rather it was either too general or too technical and too low-level to provide useful insight. I was looking for work on the HOX gene circuits and how they define cortical layers and topographic maps. There was an issue of Neuron devoted to the subject, Yuste [79], with a reference to a couple if interesting papers, see for example Dasen [14]. And just recently there was a very interesting paper in Neuron providing a transcriptional atlas of mouse neocortical layers — Belgard et al [5] — which provides some insight into the number and diversity of genes that figure in cortical development and normal function. The (next) topic however concerning molecular gradients and cortical arealization — the mechanisms that control the development of cortical areas — yielded a much richer trove of interesting work. For those new to the field, Sean B. Carroll’s [8] “Endless forms most beautiful” is probably your best bet for an introduction.
Molecular Gradients and Retinotopic Map Development [44] — Intrinsic Mechanisms in Cortical Arealization [47, 39].
The review paper — a little dated — by Mallamaci and Stoykova [41] entitled “Gene networks controlling early cerebral cortex arealization” is a good place to start. The introduction sets out the structure of the review in terms of two competing hypotheses: “This process of regional and areal differentiation of the cortical primordium is commonly termed ‘cortical arealization’. Two main models have been proposed for the cellular and molecular mechanisms controlling cortical arealization, the protomap model (Rakic, 1988) and the protocortex (or tabula rasa) model, originally put forward by Van der Loos & Woolsey (1973) and subsequently developed by O’Leary (1989). According to the former, cortical arealization would occur on the basis of molecular cues intrinsic to the cortical proliferative layer. These cues would be transferred by periventricular neural progenitors, lying in distinctive cortical regions, to their neuronal progenies, migrating along fibres of radial glia and sharing with them the same rostrocaudal and mediolateral locations. According to the latter, the cortical primordium would not have any areal bias at all. Arealization would take place on the basis of information transported to the developing cortex by subcortical afferents (mainly thalamocortical afferents). This information would be used to ‘write’ distinctive areal programs onto the cortical primordium, as if onto a clean table (hence ‘tabula rasa’). Both models are supported by very robust bodies of experimental data; this has resulted in a very heated scientific debate in this field.” And then proceeds to describe how a “synthesis of these two models has recently been achieved” and that “it is presently accepted that two main phases can be distinguished in the process of cortical arealization.”
Combinatorial Neural Circuits — Valiant [75, 76, 19].
While at times I speak of simulating a universal Turing machine using a neural circuit and an external (tape) memory, the crucial factor as Fernando Pereira points out is the depth of the combinatorial circuit you can build out of cortical structures. We are postulating that the additional stages of prenatal neurogenesis increased the depth of the combinatorial circuits you could build out of H. sapiens’ neocortex past some threshold that made possible the improvements in language, logic and decision making that gave rise to mankind’s highest achievements. On a related note, you might find it interesting to explore cases where the investigation of neural systems in various organisms has led to the development of new algorithms — see for example [1] for a recent such example.
Circuit Composition — Component Participation
In a recent conversation, Fernando mentioned Nick Pippenger in relation to Leslie Valiant’s neural modeling work. I looked up Nick’s work on “Reliable Organisms From Unreliable Components” [58] and that led me to John von Neumann’s “Theory of self-reproducing automata” [78] which happened to be on my bookshelf. Neither lead provided anything of direct relevance to my current studies, but skimming through Nick’s paper did lead me to the following observation: Most mammalian neural circuits are composed of multiple areas of the brain – often widely-separated within the brain and widely-varying in their evolutionary age — and most brain areas participate in multiple circuits. For the evolutionarily older circuits that participate in a great many circuits at the same time, it must be that they are at least modular in the sense that their contribution to each of these circuits is independent of the function of the circuits they influence. As I have mentioned elsewhere, brain functions are not reentrant. In reflecting on this observation and searching for additional evidence supporting the claim, I came across the work of Helen Mayberg who recognized a possible role for Area 25 — sometimes identified as the subgenual anterior cingulate and located under the Cingulate Cortex, see Figure 9 — in depression and a therapeutic intervention using DBS [42, 65, 63] — see Deep Brain Stimulation. Here was a somewhat mysterious, heretofore unheralded area — see Brodmann’s Area 25 — that apparently plays an important role in a circuit involving several much better known components — amygdala, hippocampus, prefrontal cortex — that ostensibly is involved in some sort of rhythmic coordination and whose pathological behavior can be interfered with in such a way to give relief to patients with the most drug- and therapy-resistant forms of clinical depression — see the popular-press articles in Wired and Scientific American. Subsequent work linked Area 25 with autism, alcohol dependence and a number of other pathologies and when functioning properly the area seems to play a role in several circuits relating to emotion perception and regulation.
Circuit Realization — Thalamo-Cortico-Striatal (TCS) Loops
Richard Granger did a piece in AI Magazine a few years back which I just happened to have buried in my stack [28] along with this shorter related work [29]. The first paper, summarizing work on algorithmic-driven analysis of cortical mechanism and function, provided some new ideas concerning how deeper combinatorial circuits might be enabled by larger brains and additional stages of neurogenesis. Here is the statement in [28] that initially caught my attention:
As the ratio of brain size to body size grows, particular allometric changes occur, defining differences between bigger and smaller brain designs. As in parallel computers, connections among components are among the most expensive attributes, strongly constraining design. As the brain grows, those structures and connection pathways that grow disproportionately large are highly likely to be the most indispensable machinery, as well as developing into the key components of human brain that may set human intelligence apart from that of other mammals. Figure 12 illustrates the three largest changes that occur:Granger cites a number of additional interesting papers that he has co-authored, e.g., Rodriguez et al [61] and Furlong et al [22], as well as numerous related papers, though not work of Valiant [74, 75] which I would have thought relevant.
Connection pathways between anterior and posterior cortex — fasciculi — grow large.
Output pathways from striatal complex change relative size: the recurrent pathway back to cortex via thalamus increases relative to the descending motor pathway.
Descending output from anterior cortex to brainstem motor systems (pyramidal tract) grows large.
These changes grow disproportionately with increased brain-body ratio, becoming notably outsized in humans.
In the section of [28] on language, he extends and elaborates on his hypothesis regarding “computational allometry:
The modeling described herein leads to a specific hypothesis: that human language arises in the brain as a function of the number of thalamo-cortico-striatal (TCS) loops. With the addition of TCS modules, some become increasingly dedicated to communication due to their inputs, just as some other areas become increasingly dedicated to particular subsets of visual inputs. Rather than wholly new brain modules that differentially process language, the evolutionary addition of TCS modules leads to the incremental acquisition of linguistic abilities. This growth need not be linear; grammars have the property of exhibiting apparently new behaviors due to the addition of just a few rules. There is a fourfold difference in overall brain size between humans and our closest primate relations (chimps, bonobos), and a far greater size difference if just the anterior cortical areas underlying language abilities are considered. There are no living apes or hominids with brain sizes between those of humans and other primates. If human language arises directly from increased TCS loops, then the present “computational allometry” argument suggests that intermediate prelinguistic or protolinguistic abilities may have been present in early hominids, even though not in extant primates. The conjecture is consistent with a broad range of constraints that are argued to rule out alternative hypotheses, e.g., Pinker 1999; Pinker & Jackendoff 2005.Tai-Sing Lee and David Mumford [37] and Dileep George and Jeff Hawkins [26] identify the layers of their hierarchical models with specific cortical areas and hence their models of the primate visual ventral pathway have layers corresponding to V1, V2, V4, IT, etc. The same goes for the earlier work by Mumford on cortico-thalamic loops [48, 49]. This is fine except that it doesn’t help the argument vis a vis why humans are cognitively more capable than non-human primates since we more or less have the same number and arrangement of cortical areas and chimps and even macaques. The work by Granger and his colleagues may not provide solid evidence for deeper combinatorial circuits, but at least it provides some complementary hypotheses and interesting conjectures about how such circuits might be implemented in the cortical — and sub-cortical in the case of the cortico-thalamic loops — substrate.
Niko Tinbergen (1907-1988) and Konrad Lorenz (1903-1989) — Fixed Action Potentials — Innate Releasing Mechanisms — see here.
Michael Shermer [68] — “The Believing Brain” — Patternicity — Adaptive Bias — Apophenia — see here and here.
Minimizing false negatives, i.e., failing to notice a risk or benefit that exists, and the tendency to infuse patterns with meaning, intention, and agency.
Paul Broca (1824-1880) — Speech Production (Broca’s Area) — Brain Function Lateralization — posterior inferior frontal gyrus of the dominant hemisphere (left in 90% of humans) — see here.
Carl Wernicke (1848-1905) — Language Comprehension (Wernicke’s Area) — superior temporal gyrus of the dominant hemisphere (left in 90% of humans) — see here.
Jerry Fodor [21] — “Modularity of Mind” — Gall’s Phrenology to Modern Cognitive Neuroscience — see here.
Modularity of mind is the notion that a mind may, at least in part, be composed of separate innate structures which have established evolutionarily developed functional purposes. As conceived by evolutionary psychologists, modules have come to be thought of as “units of mental processing that evolved in response to selection pressures” — Leda Cosmides and John Tooby [72]. Michael Gazzaniga [24] suggests that we “think of a module as a hardwired (innate) mechanism that unconsciously directs you to think or act in a certain way” and is “defined by what they do with information, not by the information they receive (the input or stimulus that triggers them).”
V.S. Ramachandran [60] — “The Tell-tale Brain” — Argument for Modularity.
Patients with a damaged Wernicke’s area but with intact Broca’s area produce elaborate, syntactically-correct sentences devoid of meaning.
In patients with a damaged Broca’s area but intact Wernicke’s area, meaning is preserved but their sentences exhibit no syntactic deep structure, e.g., no recursive embedding.
Allan Snyder — Latent Savants and repetitive Transcranial Magnetic Stimulation — see here, here and Figure 2.
V.S. Ramachandran [60] — Anterior Cingulate Cortex — Corpus Callosum “Collar” — see here.
Plays a role in a wide variety of autonomic functions, such as regulating blood pressure and heart rate, as well as rational cognitive functions, such as reward anticipation, decision-making, empathy and emotion.
Telephone Syndrome — Damage to Visual Pathway — Intact Auditory Pathway — see here.
V.S. Ramachandran [60] — “The Tell-tale Brain” — Mammalian Ossicles — see Figure 3 and Figure 4.
1. The mammalian jaw is a single bone, the mandible, while reptiles have three bones that form complex multi-hinged jaws enabling them to swallow prey whole.
2. Reptiles that lie low to the ground rest their lower jaw on the ground, so that sound transmitted through the ground is conveyed by bone conduction to the brain.
3. As they evolved into high-metabolic-rate mammals, raised off the ground and able to hunt prey on the run, they needed frequent, smaller meals to sustain their bodies and the two bones closest to the brain were re-purposed for hearing airborne sounds and the remaining bone served as hingeless, rigid jaw well adapted for tearing and chewing.
4. In each evolutionary step, the bones served an important function, and each change resulted from natural selection exploiting serendipitously convenient structures.
V.S. Ramachandran [60] — “The Tell-Tale Brain” — Babinski Sign — see here.
Reflexive withdrawal reflex causes toes to fan out and curl up in more primitive organisms, but is inhibited by the pyramidal tracts in mammals resulting in tendency to curl inward as if grasping a branch.
Eric Kandel — In Search of Memory — Aplysia gill and siphon withdrawal reflex — see here.
Stephen Jay Gould (1942-2002) — Exaptation — Preadaptation — Feathers and Flight, Mirror Neurons and Metaphor — Language — see here.
Stanislas Dehaene — Numeracy and Literacy — see here.
For two of the best books on how basic low-level faculties — in this case our ability to count and think quantitatively and our ability to recognize faces and discriminate among other complex visual stimuli — have evolved into higher-level cognitive capabilities — mathematics and reading — see Stanislas Dehaene’s [15] “The Number Sense: How the Mind Creates Mathematics” and Dehaene’s [16] “Reading in the Brain: The Science and Evolution of a Human Invention.” Dehaene is one of the clearest, most articulate and broadly informed cognitive neuroscientists I know. These two books are aimed at the general public with an interest in learning about the brain, but at the same time they are widely cited and appreciated by card-carrying neuroscientists interested in learning about new areas outside of their specific area of expertise.
Modularity in Evolutionary Biology [9] — see here.
“Evolutionary developmental biology is not yet a unified discipline, but can be distinguished from earlier approaches to evolutionary theory by its focus on a few crucial ideas. One of these is modularity: as has been long recognized, plants and animal bodies are modular: they are organized into developmentally and anatomically distinct parts. Often these parts are repeated, such as fingers, ribs, and body segments. Evo-devo seeks the genetic and evolutionary basis for the division of the embryo into distinct modules, and for the partly independent development of such modules.” The discipline of embryology is the main developmental arm of Evo-Devo. Embryologists think about how a hen’s egg transforms itself into a chick or how a human zygote transforms itself into a baby — see here. I am particularly fascinated about how basic body parts — arms, legs, torso, head — develop along with the plumbing — blood vessels, arteries — that sustains them. I find it incredible that our capillaries bring oxygenated, nutrient-rich blood to within reach of every cell in the body and carry away waste products that would otherwise kill us. From a developmental perspective, it is even more interesting to think about the afferent and efferent neurons and their axonal processes which comprise the peripheral nervous system. How do these get created and routed to location to serve their respective functions?
Sean B. Carroll [8] — Dark Matter Analogy
Carroll uses the analogy of dark matter in astrophysics to explain non-coding regions of the human genome consisting of regulatory switches. For a painless — but somewhat superficial — introduction to the science of “Evo Devo” and Carroll’s work in particular see the PBS, NOVA special here.
Sean B. Carroll [8] — some relevant excerpts from “Endless Forms Most Beautiful” (2005)
“The earliest H. sapiens fossils now known are dated at about 160,000 years old.” — Page 254
“Changes in brain size among mammals are not simply a matter of enlarging or reducing all parts of the brain proportionately. Rather, brain evolution exhibits a ‘mosaic’ pattern wth certain parts of the brain changing in concert with another, but independently of other parts.” — Page 263
“The DNA sequence of a human contains about 3 billion base pairs. In chimp DNA, about 98.8% of these bases are identical. [...] However, that 1.2% difference translates to 36 million base pairs. Because humans and chimps diverged from a common ancestor about 6 million years ago, we can assume that one-half of these difference are chimp-specific (occurred int the chimp lines) and one half human-specific (occurred in our lineage). That still leaves 18 million changes in our line since our last common ancestor. [...] Furthermore, an additional fact to consider is that any two unrelated humans will differ, on average, at about 3 million bases. [..] So, in fact, nobody knows how many changes shaped human form. My guess would be somewhere on the order of a few thousand.” — Page 269
“[C]hanges in genetic switches account for many differences in anatomical form. Because human evolution is largely a matter of the evolution of the size, shape and fine-scale anatomy of structures and of timing in development, it is only logical that switch evolution would be important in the evolution of humans as well. [...] Thus, I believe that the weight of genetic evidence is telling us that the evolution of primate, great apes, and humans is due to change more in the control of genes than in the proteins that genes encode.” — Page 270
“Still, the central importance of genetic switches to pattern formation has not fully penetrated the computational modeling world; for example see S. Wolfram, A new Kind of Science. The continuing mistake is being seduced into believing that simple rules that can generate patterns on a computer screen are the rules that generate patterns in biology.” — Page 519
Natural Selection and Fitness — Countering our Anthropocentric Bias
Natural selection doesn’t favor the smartest or the most attractive or the fastest — it favors those who in their natural environment survive to reproduce. This lesson is hard to internalize both because of our infatuation with our own abilities and because we often forget that it is the environment that shapes all organisms.
When a gene is deprecated — does not get expressed and therefore does not get the opportunity to realize its associated trait — but is retained in the genome, it tends to accumulate mutations since it isn’t having any impact on the survival and hence reproductive success of the organism.
There is an interesting idea circulating in the popular-science press that Homo economicus has subverted natural selection and substituted an alternative fitness function that favors those traits of value to culture and civilization and discounts many of the traits that were crucial in much of our most recent evolutionary history.
Limits of Evolutionary Developmental Neuroscience
What we know about the brain from Evo Devo is a very small fraction of the whole puzzle and, despite our excitement over the latest findings and the sensational stories in the popular press, in most cases the new findings are a result of stumbling on an interesting mutation in some organism other than human, finding a previously unknown gene or a known gene in a new role while investigating a particular deficit or disease, or literally stumbling over the bones — fossil record — of one odd organism in one particular evolutionary branch, the full extent of which we’ll probably never know. Sean B. Carroll makes a similar point intended to caution scientists not to over dramatize their latest results for the simple reason that doing so often results in sensational, over-hyped coverage in the press and the inevitable disappointment — and popular disillusionment in scientific “progress” — when subsequent new evidence forces us to reconsider earlier our claims and revise our theories.
Dynamic Reorganization of Rat Somatosensory Cortex — see Figure 5 and Figure 6.
Nicolelis [51] presents evidence for dynamic, distributed neural representations, contrasting with primarily static, local representations that he disparagingly associates with Franz Gall and phrenology and that he claims dominated neuroscience for much of its history. If we accept that inference in the brain is carried out in a distributed fashion and that memories are encoded in a population of cells, then it seems we need a mechanism whereby ensembles of neurons — possibly at some distance from one another — can be coordinated during learning and inference. Neuronal oscillations have been suggested as one possible mechanism. This paper examines the possible role of local field potentials (LFP) in enabling such coordinated spiking and, in particular, LFP–LFP coupling across distal brain areas — see Canolty et al [7] which was suggested to me by one of the authors Charles Cadieu. They show that “spiking activity in single neurons and neuronal ensembles depends on dynamic patterns of oscillatory phase coupling between multiple brain areas, in addition to the effects of proximal LFP phase. Neurons that prefer similar patterns of phase coupling exhibit similar changes in spike rates, whereas neurons with different preferences show divergent responses, providing a basic mechanism to bind different neurons together into coordinated cell assemblies.” — see Figure 11.
Waterfall Optical Illusion
Stare at a waterfall for 30 seconds then look away at some rocks; the rocks appear to be rising. The explanation given is that different populations of neurons are firing, one in response to downward motion and a second in response to upward motion; the ones for downward motion get “tired” or adapted to the predominant motion. Even in the absence of movement there is some spontaneous firing. Thus when you start at the stationary rocks, the downward responsive neurons have been damped and the spontaneous firing of the upward responsive neurons have a temporary advantage since the usual balance of power — both populations spontaneously firing at about the same rate — has shifted.
The Shift from Local to Distant Functional Connectivity During Primate Development [17, 66] — see here.
Changes in the quantity of grey matter (neurons, neuropil — dendrites and unmyelinated axons, glial cells) and white matter (myelinated axons) in the period from 6 to 25 years of age. Back to front, unused axon die off coupled with myelination, plus connections between the frontal cerebral cortex the cerebellar cortex. This cerebral-cerebellar pathway — and evidence of a cerebellar role in language — relieves some of the pressure on the Single Algorithm hypothesis.
Michael Gazzaniga [24] — “Human”
The differences between the cortex in humans and non-human primates can summarized by the following: we both start with the same basic types of neurons Nimchinsky et al [52], some functional areas have more neurons, some layers of some areas — in particular prefrontal areas — are more densely packed, we are both stuck with the same number connections per neuron, however, this encourages specialization, and, in particular, more intra-regional — within region — connections and faster intra-regional — between selective pairs of regions — connections. Here are some relevant excerpts from [24]:
Although the number of neurons increases, they cannot increase the absolute number of connections each one makes. What tends to happen is that, as absolute brain size increases, the proportional connectivity decreases. [...] Fewer dense connections force the brain to specialize, create local circuits, and automate. [...] Is the cortex evenly enlarged, or are some parts preferentially enlarged, and if so, which ones. Let’s start with the occipital lobe, which contains among other things, the primary visual or striate, cortex. In chimps, it constitutes 5% of the entire neocortex, whereas in humans it constitutes 2%, which is less than would be expected. [...] The striate cortex in fact is the exact size that it is predicted to be for an ape of our size. [...] The frontal lobe, until recently, was thought to be proportionally larger in humans that other primates. [...] Katerina Semendeferi and colleagues [64] published a study [...] concluded that humans do not have a larger frontal lobe that expected for a primate with their brain size. [...] Preuss [59] argues that even if you accept that the frontal lobes did not expand out of proportion to the rest of the cortex, a distinction should be made between the frontal and prefrontal cortex. [...] In fact, Semendeferi confirmed that area 10, in the lateral prefrontal cortex, is almost twice as large in humans as in apes. Area 10 is involved with memory and planning, cognitive flexibility, abstract thinking, initiating appropriate behavior and inhibiting inappropriate behavior, learning rules, and picking out relevant information from what is perceived through the senses. [...] Schoenemann and colleagues [...] round that the prefrontal white matter was disproportionately larger in humans that other primates and concluded that this suggests a higher degree of connectivity in this part of the brain. [...] The prefrontal cortex is interesting in another way. Non-primate mammals have two major regions of the prefrontal cortex, and primates have three. [...] The new region tacked onto [the orbital prefrontal region which responds to rewarding external stimuli and the anterior cingulate cortex which processes information about the body’s internal state, both thereby contributing to the emotional aspects of decision making] is called the lateral or granular prefrontal cortex, and it is where area 10 is. This new region is apparently unique to primates and is concerned mainly with the rational aspects of decision making, which are our conscious efforts to reach a decision. This region is densely inteconnecgted with other regions that are larger in human brains — the posterior parietal cortex and temporal lobe cortex — and outside the neocortex, it is connected to several cell groups in the dorsal thalamus that are also disproportionately enlarged, the medial dorsal nucleus and pulvinar. [Page 18–22]
Primates have more cortical areas than other mammals. It has been found that they have nine or more premotor areas, whereas non-primates have only two to four. It is tempting to think that because we humans are higher functioning, we would have more cortical areas than other primates. Indeed, very recent evidence indicates that unique areas have been found in the visual cortex of the human brain. David Heeger at New York University has just discovered these new areas which hare not found in other primates. For the most part, however, additional cortical areas have not been found in humans. [...] Besides the fact that there is no evidence have radically more cortical areas than apes, there is increasing evidence that there are equivalent cortical areas in apes for human-specific functions. It appears that other primates, not just the great apes, also have cortical areas that correspond to our language areas and tool-use areas., and that these areas are also lateralized , meaning that they are found predominately in one hemisphere rather than the other, just as they are in humans. [...] What has been found to be unique within the human brain is in an area called the planum temporale, which all primates have. This is a component of Wernicke’s area, the cortical area associated with language input, such as the comprehension of both written and spoken language. The planum temporale is larger on the left side that the right side in humans, chimps and rhesus monkeys, but it is microscopically unique in the left hemisphere of the humans. Specifically what is different is that the cortical minicolumns of the planum temporale are larger and the area between the columns is wider on the left of of the human brain than on the right side, while in chimps and rhesus monkeys the columns and the inter-columnar spaces are the same size on both sides of the brain. [Page 22–25]
Several scientists have suggested that the supragranular layers, and the networks of connections they form between the cortical locations, participate heavily in higher cognitive functions. This is accomplished by linking motor, sensory and association areas. These areas receive sensory inputs from high-order sensory systems, interpret them in the light of similar past experiences, and function in reasoning, judgement, emotions, verbalizing ideas, and storing memory. [...] The horizontal expansion of the cortical sheet [...] and alterations to the basic structure of cortical columns are likely determined early in fetal development by altering the number and timing of cell divisions that generate cortical neurons. Cortical neurogenesis can be divided into an early and a late period. The length of time and number of cell cycles spent in the early period of cell division will ultimately determine the number cortical columns that will be found in any given species, The length of time and the number of cell cycles spent in the later period may determine the number of individual neurons within a cortical column. A higher number of early divisions will result in a larger cortical sheet and a higher number of later divisions will resulting a higher number of neurons within an individual column. [Page 25–17]
Gazzaniga also cites evidence of differences between human and ape mirror neurons. According to Gallese et al [23] the human motor neuron system responds to a wider range of actions. In monkeys, the presence of the object — in, say, the context of grasping — appears to be necessary to enable motor neuron activation, whereas mimed actions suffice to cause firing in humans. Just prior to my presentation at the Machine Perception Tech Talk I received a message from Gal Chechik in which he mentioned:
I think it would be fair to say that our understanding of genetic regulation of development in primates is “very very” limited. As you probably know, the first large scale data on human transcriptome in brain development was just published three weeks ago, but I am not aware of similar developmental data in monkeys. So although we know which genes are different between monkeys and humans, we still need to map where and when they are expressed in the brain.I’m in complete agreement with Gal and the more I get into this area the more confusion and disagreement I encounter. The paper he’s referring to is Kang [33] and is available here. Another even more recent relevant paper is Flight [20] and is available here.
What can Holothuria glaberrima (Daniel Wolpert’s sea cucumber), Caenorhabditis elegans (Brenner’s marine worm), Aplysia californica (Eric Kandel’s sea slug), Rana pipiens (Lettvin and Maturana’s frog), and Rattus norvegicus (Nicolelis’ rats) can tell us about Homo sapiens?
The sea cucumber starts off as a mobile life form before attaching itself to a rock and digesting its brain; Wolpert’s lesson is that you don’t need a brain if you can’t move.
C. elegans nervous system consists of 302 non-spiking neurons each one a highly specialized analog computer; this isn’t the way to go if you want to scale a brain to billions of neurons — see Figure 13.
Charles Sherrington wrote the book on muscle reflex circuits but Eric Kandel’s work on the gill-siphon reflex showed how fundamental were the basic neural processes underlying learning; sensing and acting are tightly coupled at all levels of the nervous system.
Lettvin, Maturana, McCulloch and Pitts [38] demonstrated how the periphery — eye — and the brain — specificlaly the tectum which is the precursor to the cortex in mammals — share the responsibility for perception; evolutionary pressure encouraged encephalization which made possible sensory fusion and provided an opportunity for a more general computational substrate.
Research on the representation information from the rat whisker pad in the primary somatosensory cortex showed that topographic map formation is highly plastic; the lesson being that the machinery for building representations in primary sensory cortex is adaptively keyed to environmental changes.
You can think of the above lessons as the use cases which I try to keep in mind when I attempt to reverse engineer the brain.
In Defense of the Single Algorithm Hypothesis
There are certainly plenty of dimensions along which to differentiate cells and circuits belonging to the 100 or so different functional areas in the cortex: the histlogy of individual cells, the cytoarchitecture of cell complexes, neurotransmitters, genetic pathways, connections to thalamus, cerebellum, etc., even morphology-specific-frequency-modulated plasticity [36].
Even so, none of this precludes the possibility that all of these component areas are running the same algorithm — they may have different implementations and the unifying algorithm may be applied to different data, but these differences are not algorithmic.
The variations that distinguish components may be necessary to orchestrate their simultaneous application to different data thereby allowing parallelism.
I have heard it said by a number of computer scientists and computational neuroscientists that the genome is not big enough — does not encode enough information — to completely specify the structure of the cerebral cortex and therefore it is necessary to learn this structure in an unsupervised manner. I am sympathetic to the notion that much of the cortex fine-grained connectivity is established through unsupervised learning; however, it is important to keep in mind that the genome doesn’t have to specify every connection explicitly. There is considerable evidence to suggest that a great deal of the structure of the cortex is determined by complex genomic regulatory networks that use a variety of standard developmental machinery, e.g., molecular gradient following, area-based regulatory switching, to implement program that induce extremely complex structure on the developing cortex [47, 39, 41].
In Defense of the Quantitative Sufficiency Hypothesis
The cortical coprocessor model — Suppose that there are no or very few cortical areas that are present in humans but not in our closest primate relatives and that each area is histologically and cytoarchitecturally homogeneous. Also suppose that the intra-areal and extra-cortico connections in humans are also realized in our closest relatives in kind if not in quantity. In this case it would be relatively simple for additional rounds of cell division during fetal brain development to exploit local chemical gradients and cell-differentiation signals to proportionally expand the cortex.
This is analogous to how semiconductor designers take advantage of new fabrication technologies to improve products without making significant changes to the design. As it becomes feasible to print more transistors on a single die, you can increase the number of processing cores, SIMD lanes, cache sizes, etc., with only modest changes to the overall design.
It would be interesting to find a study comparing the maturation of mammalian brains relative to the normalized volume of both white and grey matter.
Summary Positions Regarding Three Controversial Hypotheses
Executive Summary:
Structural (morphological) modularity at the genomic level enables computational scaling just as modular circuit designs enable computational scaling in modern computer architectures.
The level of granularity due to algorithmic parsimony if such a principle can be said to apply to the cortex is probably more than a logic gate but how much more so is open to debate.
Additional stages of prenatal neurogenesis could plausibly increase the depth of combinatorial neural circuits thus facilitating longer chains of inference and deeper recursive embedding.
Modularity of Mind Hypothesis (Summary)
The accretive process of evolution — especially more recent hominin1 evolution — isn’t likely to yield physiological locality of function, though Fodor’s requirement of “fixed neural architecture” does not seem to require physical localizability [21].
Moreover, even if we dispense with the requirement of structural locality, the idea of a circumscribed cognitive faculty seems at odds with what we know about functional areas in cortex for all but the most narrow of capacities [57]. Informational encapsulation in which specific competences do not have to appeal to other cognitive modules seem rare.
Even if high-level cognition is not modular, the neural substrate on which it depends does appear to be highly modular in its construction — in the engineering sense of the structure of the brain being divided into parts that can be developed and operated independently. Finally, while there may be some advantage to the alternative idea of domain-general processing in which mental activity cannot be divided across independent units [73], this does not seem to reflect our functional or behavioral understanding of high-level cognitive capacities in primates with the possible exception of very late (evolutionarily speaking) additions to our cognitive repertoire.2
Single Algorithm Hypothesis (Summary)
The argument appealing to cytological variation among Brodmann’s areas speaks to variability in the neural implementation and not to algorithmic diversity.
The experiments re-wiring ferret brains [50] and the ubiquity of topological map formation among primary sensory and motor cortex provides a compelling case for some core algorithmic principals at play at least in these evolutionarily more recent cortical areas — but see [2] for a characterization of the significant cytoarchitectural differences between auditory and visual cortex.
Connections from cortical areas implicated in decision making, language, speech, movement execution and planning to motor and sequence machinery in thalamic nuclei and the cerebellar cortex call for a more complicated set of algorithmic principals than I expect Hinton, DiCarlo and Lewicki had in mind. This also applies to evolutionarily older core including the posteromedial cortices which are implicated in creating the rich associations involved in the autobiographical self [56, 12].
Quantitative Sufficiency Hypothesis (Summary)
The simplicity of the “three additional round of cell division during fetal brain development” belies the complexity and robust integrative capacity of fetal development.
As a species we are biased to think that our cognitive capacities are well beyond the apes we see in the wild or in zoos — as opposed to those few raised in captivity in a rich environment interacting almost constantly with humans.
We tend to exaggerate or overemphasize our innate individual capacities and downplay the benefit we derive from civilization including written and spoken language, various affordances for augmenting our finite short and long term memory, and the readily available sources of knowledge available through books, libraries and now the Internet.
It seems reasonable to assume given what we know about the physiology that differences in cognitive capacity between apes and humans are — as Darwin suggested — one of degree and not of kind.3 Moreover the basic cytoarchitecture of the neocortex — cortical columns — and the mechanisms for expanding the cortical sheet in primates appear to be scalable along all the key dimensions of computation.4 The combination of a scalable computational architecture and bootstrapping cultural affordances — from paper and pencil to books and computers — to overcome memory limitations seems adequate to the job of explaining the observed differences between the capabilities of humans and their closest primate cousins. What do you get with deeper combinatorial circuits?
Recursive embedding to create more complicated linguistic structure;
Deeper theories of mind about how agents think about other agents;
Managing with large cliques and more complicated social situations;
More complicated physical theories and sophisticated causal models;
Deeper layers of abstraction, compositional models and motor neurons;
Supplementary Material Including Figures and Bibliography
Figure 1: Two drawings of cortical lamination by Santiago Ramon y Cajal, each showing a vertical cross-section, with the surface of the cortex at the top. Left: Nissl-stained visual cortex of a human adult. Right: Nissl-stained motor cortex of a human adult — see here for detail.
 |
Figure 2: A three-year-old child named Nadia became famous for her ability to sketch spectacularly detailed horses and riders from memory. Savants like Nadia show the ability to perform unusual feats of illustration or calculation when they are younger than 6 — see here for background and more drawings.
 |
Figure 3: The ossicles — malleus (hammer), incus (anvil) and stapes (stirrup) — are the three smallest bones in the human body. They are contained within the middle ear space and serve to transmit sounds from the air to the fluid-filled labyrinth (cochlea).
 |
Figure 4: Reptile hinged jaw — The snake and its ancestor the mosasaur have a hinged jaw that enables them to swallow their prey whole. The bones that comprise the intermediate links in the hinge are believed to have evolved into the small bones that are part of the mammalian auditory system.
 |
Figure 5: Michigan and Utah (pictured) microwire electrode arrays.The Michigan arrays allow a higher density of sensors for implantation as well as a higher spatial resolution than microwire MEAs. They also allow signals to be obtained along the length of the shank, rather than just at the ends of the shanks. In contrast to Michigan arrays, Utah arrays are 3-D, consisting of 100 conductive silicon needles. However, in a Utah array signals are only received from the tips of each electrode, which limits the amount of information that can be obtained at one time. Furthermore, Utah arrays are manufactured with set dimensions and parameters while the Michigan array allows for more design freedom — see here.
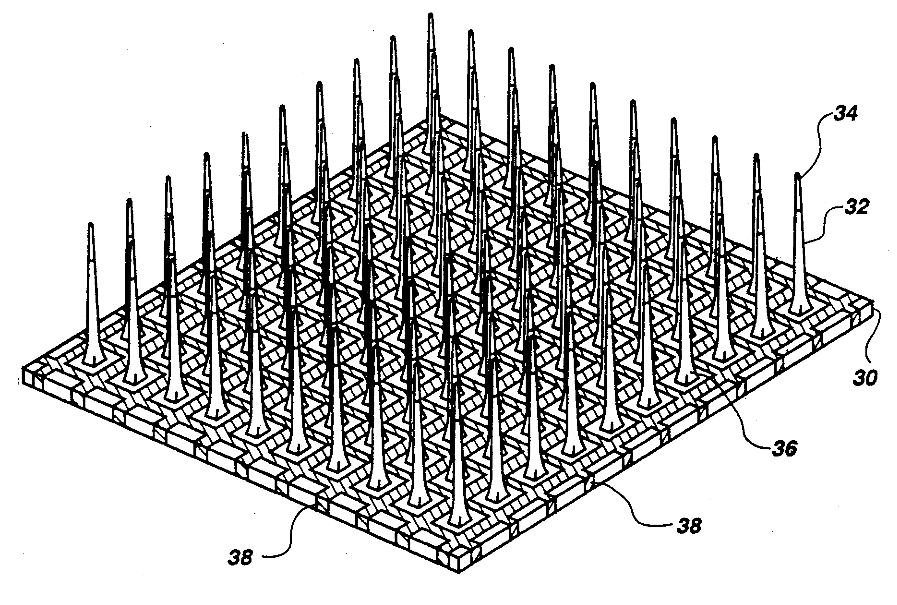 |
Figure 6: Nicolelis [51] and his colleagues developed a model of the rat somatosensory system which includes feed-forward connections from the periphery — via ascending trigeminothalamic projections — to the ventroposterior medial thalamus (VPM) and feed-back from the cortex — via descending corticofugal projections — to the primary somatosensory cortex (S1).
 |
Figure 7: Autonomic Nervous System (ANS): Sympathetic (SNS) and Parasympathetic (PSNS) Systems — The ANS is responsible for regulation of internal organs and glands, which occurs unconsciously. The SNS and PSNS complement one another and work in relative opposition — simplifying a good deal — in the regulation of internal organs and glands. The general action of the SNS is to mobilize the body’s nervous system “fight-or-flight” response. It is, however, constantly active at a basal level to maintain homeostasis. The PSNS is responsible for stimulation of “rest-and-digest” activities that occur when the body is at rest, including sexual arousal, salivation, lacrimation (tears), urination, digestion and defecation. — caption text excerpted from here and here and figure graphic borrowed from [4]
 |
Figure 8: Schematic flowchart of human brain development — see here for more detail.
 |
Figure 9: Limbic-cortical dysregulation model [65]. Anatomical interconnections are grouped into four main behavioral compartments. Abbreviations: mF = medial prefrontal; aCg = rostral anterior cingulate; oF = orbital frontal; cd-vs = caudate-ventral striatum; thal = thalamus; mb-p = midbrain-pons; Cg25 = subgenual cingulate; a-ins = anterior insula; am = amygdala, hth = hypothamus; bs = brainstem; PF = dorsolateral prefrontal; p = parietal; pCg = posterior cingulate. Numbers: Brodmann designations.
 |
Figure 10: In the nucleus during transcription, the cell’s machinery copies the gene sequences into messenger RNA (mRNA). Like DNA, mRNA has four nucleotide bases, but in mRNA the base urocil (U) replaces thymine (T). The nucleus also creates transfer RNA (tRNA) which is used to transport amino acids to the ribosomes and ribosomal RNA (rRNA) which is used to construct ribosome-specific proteins. The mRNA, tRNA and rRNA are small enough travel through the nucleus membrane into the cytoplasm. During translation, protein-constructing ribosomes read the mRNA sequences and translate them into amino acid sequences. The ribosome begins at the “start” sequence AUG, then reads three nucleotides at a time. Each three-nucleotide codon specifies a particular amino acid. The “stop” codons (UAA, UAG and UGA) tell the ribosome when the full sequence is complete. Once complete the sequence still needs to be folded into a particular conformation in order to serve its purpose in the organism.
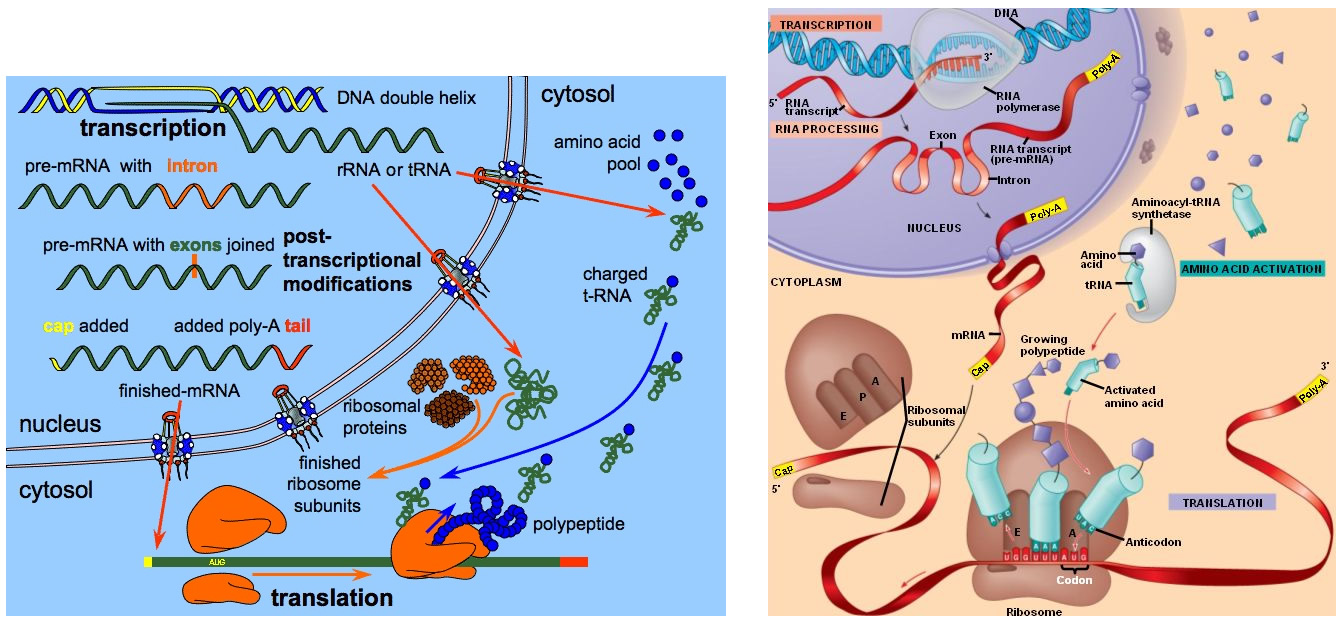 |
Figure 11: Patterns of oscillatory phase coupling across multiple brain areas coordinate anatomically dispersed neuronal cell assemblies (schematic). (A–D) Hypothesis 1: Spike timing in single neurons depends on frequency-specific oscillatory phase coupling across multiple brain areas. (A) Spiking in one area may depend on population activity (local field potentials, LFPs) occurring in multiple areas. (B) Many neurons are sensitive to oscillatory LFP activity occurring in particular frequency bands; filtering all LFPs at this frequency and extracting phases can reveal patterns of phase coupling between LFP channels. (C) The strength of LFP–LFP phase coupling is different for spike times compared with randomly selected times and defines a neuron’s preferred pattern of LFP–LFP phase coupling, similar to a receptive field. That is, when LFP activity matches the neuron’s preferred pattern of LFP–LFP phase coupling, the cell spikes more often. (D) Given novel LFP phases as input, the model generates a predicted coupling-based spike rate output, which can then be compared with the measured spike rate. (E–G) Hypothesis 2: Large-scale patterns of phase coupling synchronize anatomically dispersed neuronal ensembles. (E) The procedure described above can be applied to multiple simultaneously recorded neurons. (F) Cells that prefer similar LFP–LFP phase-coupling patterns exhibit similar coupling-based rates. (G) Shared variability in coupling-based rates is compactly described by a single phase coupling network that defines a cell assembly. That is, it is possible to identify large-scale patterns of LFP–LFP phase coupling (G) that explain a significant fraction of the variation in spike rates for a large ensemble of neurons distributed across multiple brain areas. (H–J) Hypothesis 3: Differential sensitivity to distinct brain rhythms or coupling patterns permits selective control of multiple coactive assemblies. (H) Multiple functional ensembles, each spanning several brain areas, overlap in space. (I) Interference between ensembles is minimized when each assembly responds to a different frequency (assemblies A and C) or distinct phase-coupling pattern (assemblies A and B). (J) Frequency and pattern selectivity permits dynamic, independent coordination of multiple coactive ensembles. (Credit: Figure 1 in Canolty et al [7])
 |
Figure 12. Allometric changes in primary components of telencephalon. The anatomical connection pathways among posterior and anterior neocortex (PC, AC), striatum (S), and pallidum (P) are shown for small-brained (a) and large-brained (b) mammals. Sensory inputs (vision, audition, touch) arrive at thalamus (T); projection loops connect thalamus with cortex and cortex to striatum to pallidum and back to thalamus; both pallidal and motor cortex efferents target brainstem motor nuclei (dashed box). (a) In small-brained mammals, primary output from pallidum is to motor systems; primary output of anterior cortex is to striatum. (b) Prominent allometric connection changes in large-brained mammals: (1) Substantial growth occurs in projections between anterior and posterior cortical regions (fasciculi). (2) Pallidal outputs increasingly target thalamus, completing the large cortico-striatal-thalamo-cortical loops. (3) Anterior cortical projections to motor targets grow large (pyramidal tract). — from [28] and [29]
 |
Figure 13. The connectome for C. Elegans; its nervous system consists of 302 non-spiking neurons each one a highly specialized analog computer.
 |
References
| [1] |
Yehuda Afek, Noga Alon, Omer Barad, Eran Hornstein, Naama Barkai, and Ziv
Bar-Joseph.
A biological solution to a fundamental distributed computing problem.
Science, 331(6014):183–185, 2011.
(PDF)
|
| [2] |
K. Amunts, A. Schleicher, and K. Zilles.
Cytoarchitecture of the cerebral cortex — more than localization.
NeuroImage, 37(4):1061–1065, 2007.
(PDF)
|
| [3] |
J. H. Balsters, E. Cussans, J. Diedrichsen, K. A. Phillips, T. M. Preuss, J. K.
Rilling, and N. Ramnani.
Evolution of the cerebellar cortex: The selective expansion of
prefrontal-projecting cerebellar lobules.
NeuroImage, 49(3):2045–2052, 2010.
(PDF)
|
| [4] |
Mark F. Bear, Barry Connors, and Michael Paradiso.
Neuroscience: Exploring the Brain (Third Edition).
Lippincott Williams & Wilkins, Baltimore, Maryland, 2006.
|
| [5] |
T. Grant Belgard, Ana C. Marques, Peter L. Oliver, Hatice Ozel O. Abaan,
Tamara M. Sirey, Anna Hoerder-Suabedissen, Fernando García-Moreno,
Zoltán Molnár, Elliott H. Margulies, and Chris P. Ponting.
A transcriptomic atlas of mouse neocortical layers.
Neuron, 71(4):605–616, 2011.
(PDF)
|
| [6] |
P. Callaerts, G. Halder, and W. J. Gehring.
PAX-6 in development and evolution.
Annual Review of Neuroscience, 20:483–532, 1997.
(PDF)
|
| [7] |
Ryan T. Canolty, Karunesh Ganguly, Steven W. Kennerley, Charles F. Cadieu,
Kilian Koepsell, Jonathan D. Wallis, and Jose M. Carmena.
Oscillatory phase coupling coordinates anatomically dispersed
functional cell assemblies.
Proceedings of the National Academy of Sciences,
107(40):17356–17361, 2010.
(PDF)
|
| [8] |
Sean B. Carroll.
Endless forms most beautiful: the new science of evo devo and
the making of the animal kingdom.
W.W. Norton & Co., 2005.
|
| [9] |
V.A. Casagrande and G. Purushothaman.
Modularity.
In E. Bruce Goldstein, editor, Encyclopedia of Perception,
Volume 1, pages 561–566. Sage Publications, Thousand Oaks, CA, 2009.
(PDF)
|
| [10] |
Chin Chiang, Ying Litingtung, Eric Lee, Keith E. Young, Jeffrey L. Corden,
Heiner Westphal, and Philip A. Beachy.
Cyclopia and defective axial patterning in mice lacking sonic
hedgehog gene function.
Nature, 383:407–413, 1996.
(PDF)
|
| [11] |
M. Christensen, A. Estevez, X. Yin, R. Fox, R. Morrison, M. McDonnell,
C. Gleason, D.M. Miller 3rd, and K. Strange.
A primary culture system for functional analysis of c. elegans
neurons and muscle cells.
Neuron, 33(4):503–14, 2002.
(PDF)
|
| [12] |
Antonio Damasio.
Self Comes to Mind: Constructing the Conscious Brain.
Albert A. Knopf, New York, NY, 2010.
|
| [13] |
Charles Darwin.
The Origin of Species by Means of Natural Selection, or the
Preservation of Favoured Races in the Struggle for Life.
John Murray, London, 1859.
|
| [14] |
J.S. Dasen, J. Liu, and T.M. Jessell.
Motor neuron columnar fate imposed by sequential phases of Hox-c
activity.
Nature, 425(6961):926–933, 2003.
(PDF)
|
| [15] |
Stanislas Dehaene.
The Number Sense: How the Mind Creates Mathematics.
Oxford University Press, 1999.
|
| [16] |
Stanislas Dehaene.
Reading in the Brain: The Science and Evolution of a Human
Invention.
Viking Press, 2009.
|
| [17] |
D.A. Fair, A.L. Cohen, J.D. Power, N.U.F. Dosenbach, and J.A. Church.
Functional brain networks develop from a local to distributed
organization.
PLoS Computational Biology, 5(5), 2009.
(PDF)
|
| [18] |
Dean Falk.
Primate evolution and human origins.
In W. Henke, H. Rothe, and I. Tattersall, editors, Handbook of
Palaeoanthropology, Volume 2, pages 1133–1162. Springer Verlag, 2007.
(PDF)
|
| [19] |
Vitaly Feldman and Leslie G. Valiant.
Experience-induced neural circuits that achieve high capacity.
Neural Computation, 21(12):2715–2754, 2009.
(PDF)
|
| [20] |
Monica Hoyos Flight.
Gene expression: The dynamics of the brain transcriptome revealed.
Nature Review Neuroscience, 12:703–703, 2011.
|
| [21] |
J. Fodor.
Modularity of Mind.
MIT Press, Cambridge, Massachusetts, 1984.
|
| [22] |
Jeff Furlong, Andrew Felch, Jayram Moorkanikara Nageswaran, Nikil Dutt, Alex
Nicolau, Alexander V. Veidenbaum, Ashok Chandrashekar, and Richard Granger.
Novel brain-derived algorithms scale linearly with number of
processing elements.
In Proceedings of the International Conference on Parallel
Computing, pages 767–776, 2007.
(PDF)
|
| [23] |
Vittorio Gallese, Christian Keysers, and Giacomo Rizzolatti.
A unifying view of the basis of social cognition.
Trends in Cognitive Sciences, 8:396–403, 2004.
|
| [24] |
Michael S. Gazzaniga.
Human.
Harper Collins, New York, 2008.
|
| [25] |
Michael S. Gazzaniga.
The Cognitive Neurosciences (Third Edition).
Bradford Books. MIT Press, Cambridge, MA, 2009.
|
| [26] |
Dileep George and Jeff Hawkins.
Towards a mathematical theory of cortical micro-circuits.
PLoS Computational Biology, 5(10), 2009.
(PDF)
|
| [27] |
J. N. Giedd, J. Blumenthal, N. O. Jeffries, F. X. Castellanos, H. Liu,
A. Zijdenbos, T. Paus, A. C. Evans, and J. L. Rapoport.
Brain development during childhood and adolescence: a longitudinal
MRI study.
Nature Neuroscience, 2(10):861–863, 1999.
(PDF)
|
| [28] |
Richard Granger.
Engines of the brain: the computational instruction set of human
cognition.
AI Magazine, 27:15–32, 2006.
(PDF)
|
| [29] |
Richard Granger.
The evolution of computation in brain circuitry [excerpt from review
of striedter 2005].
Behavioral and Brain Sciences, pages 17–18, 2006.
(PDF)
|
| [30] |
Viren Jain, H. Sebastian Seung, and Srinivas C. Turag.
Machines that learn to segment images: a crucial technology for
connectomics.
Current Opinion in Neurobiology, 20(5):1–14, 2010.
(PDF)
|
| [31] |
Mark H. Johnson.
Functional brain development in infants: Elements of an interactive
specialization framework.
Child Development, 71(1):75–81, 2001.
(PDF)
|
| [32] |
E.R. Kandel, J.H. Schwartz, and T.M. Jessell.
Principles of neural science (Fourth Edition).
McGraw-Hill, Health Professions Division, 2000.
|
| [33] |
Hyo Jung Kang, Yuka Imamura Kawasawa, Feng Cheng, Ying Zhu, Xuming Xu, Mingfeng
Li, Andre M. M. Sousa, Mihovil Pletikos, Kyle A. Meyer, Goran Sedmak, Tobias
Guennel, Yurae Shin, Matthew B. Johnson, Zeljka Krsnik, Simone Mayer, Sofia
Fertuzinhos, Sheila Umlauf, Steven N. Lisgo, Alexander Vortmeyer, Daniel R.
Weinberger, Shrikant Mane, Thomas M. Hyde, Anita Huttner, Mark Reimers,
Joel E. Kleinman, and Nenad Sestan.
Spatio-temporal transcriptome of the human brain.
Nature, 478:483–489, 2011.
|
| [34] |
J.N. Kay, I. De la Huerta, I.J. Kim, Y. Zhang, M. Yamagata, M.W. Chu,
M. Meister, and J.R. Sanes.
Retinal ganglion cells with distinct directional preferences differ
in molecular identity, structure, and central projections.
Journal of Neuroscience, 25(31):7753–7762, 2011.
(PDF)
|
| [35] |
K.N. Kay, T. Naselaris, R.J. Prenger, and J.L. Gallant.
Identifying natural images from human brain activity.
Nature, 452:352–355, 2008.
(PDF)
|
| [36] |
Arvind Kumar and Mayank R. Mehta.
Frequency dependent changes in NMDAR-dependent synaptic plasticity.
Frontiers in Computational Neuroscience, 5(0), 2011.
(PDF)
|
| [37] |
Tai Sing Lee and David Mumford.
Hierarchical Bayesian inference in the visual cortex.
Journal of the Optical Society of America, 2(7):1434–1448,
2003.
(PDF)
|
| [38] |
J. Y. Lettvin, H. R. Maturana, W. S. McCulloch, and W. H. Pitts.
What the frog’s eye tells the frog’s brain.
Proceedings of the Institute for Radio Engineers,
47:1940–1951, 1959.
(PDF)
|
| [39] |
Michael Levine and Eric H. Davidson.
Gene regulatory networks for development.
Proceedings of the National Academy of Sciences of the United
States of America, 102(14):4936–4942, 2005.
(PDF)
|
| [40] |
Philip Lieberman.
On the nature and evolution of the neural bases of human language.
American Journal of Physical Anthropology, 119(S35):36–62,
December 2002.
(PDF)
|
| [41] |
Antonello Mallamaci and Anastassia Stoykova.
Gene networks controlling early cerebral cortex arealization.
European Journal of Neuroscience, 23(4):847–856, 2006.
(PDF)
|
| [42] |
H. S. Mayberg, M. Liotti, S. K. Brannan, S. McGinnis, R. K. Mahurin, P. A.
Jerabek, J. A. Silva, J. L. Tekell, C. C. Martin, and J. L. Lancaster.
Reciprocal limbic-cortical function and negative mood: converging
PET findings in depression and normal sadness.
The American Journal of Psychiatry, 156(5):675–82, 1999.
(PDF)
|
| [43] |
W. McGinnis, R. L. Garber, J. Wirz, A. Kuroiwa, and W. J. Gehring.
A homologous protein-coding sequence in drosophila homeotic genes and
its conservation in other metazoans.
Cell, 37(2):403–408, 1984.
|
| [44] |
T. Mclaughlin and D. D. O’Leary.
Molecular gradients and development of retinotopic maps.
Annual Review of Neuroscience, 28(1):327–355, 2005.
(PDF)
|
| [45] |
T. M. Mitchell, S. V. Shinkareva, A. Carlson, K.M. Chang, V. L. Malave, R. A.
Mason, and M. A. Just.
Predicting human brain activity associated with the meanings of
nouns.
Science, 320(5880):1191–1195, 2008.
(PDF)
|
| [46] |
Vernon Mountcastle.
An organizing principle for cerebral function: the unit model and the
distributed system.
In Gerald Edelman and Vernon Mountcastle, editors, The Mindful
Brain, pages 7–50. MIT Press, Cambridge, MA, 1978.
|
| [47] |
Sven Mühlfriedel, Friederike Kirsch, Peter Gruss, Kamal Chowdhury, and
Anastassia Stoykova.
Novel genes differentially expressed in cortical regions during late
neurogenesis.
European Journal of Neuroscience, 26(1):33–50, 2007.
|
| [48] |
David Mumford.
On the computational architecture of the neocortex I: The role of
the thalamo-cortical loop.
Biological Cybernetics, 65:135–145, 1991.
(PDF)
|
| [49] |
David Mumford.
On the computational architecture of the neocortex II: The role of
cortico-cortical loops.
Biological Cybernetics, 66:241–251, 1992.
(PDF)
|
| [50] |
Jessica R. Newton and Mriganka Sur.
Rewiring cortex: Functional plasticity of the auditory cortex during
development.
In J. Syka and M. M. Merzenich, editors, Plasticity of the
central auditory system and processing of complex acoustic signals, pages
127–138. Springer Verlag, 2004.
(PDF)
|
| [51] |
Miguel Nicolelis.
Beyond Boundaries: The New Neuroscience of Connecting Brains
with Machines — and How It Will Change Our Lives.
Henry Holt and Company, 2011.
|
| [52] |
Esther A. Nimchinsky, Emmanuel Gilissen, John M. Allman, Daniel P. Perl,
Joseph M. Erwin, and Patrick R. Hof.
A neuronal morphologic type unique to humans and great apes.
Proceedings of the National Academy of Sciences,
96(9):5268–5273, 1999.
(PDF)
|
| [53] |
Shinji Nishimoto, An T. Vu, Thomas Naselaris, Yuval Benjamini, Bin Yu, and
Jack L. Gallant.
Reconstructing visual experiences from brain activity evoked by
natural movies.
Current Biology, 0:000–000, 2011.
(PDF)
|
| [54] |
Christiane Nusslein-Volhard and Eric Wieschaus.
Mutations affecting segment number and polarity in drosophila.
Nature, 287:795–801, 1980.
(PDF)
|
| [55] |
Randall C. O’Reilly.
Biologically based computational models of high-level cognition.
Science, 314(5796):91–94, 2006.
(PDF)
|
| [56] |
Josef Parvizi, Gary W. Van Hoesen, Joseph Buckwalter, and Antonio Damasio.
Neural connections of the posteromedial cortex in the macaque.
Proceedings of the National Academy of Sciences of the United
States of America, 103(5):1563–1568, 2006.
(PDF)
|
| [57] |
Stephen Pinker.
How the Mind Works.
W. W. Norton, New York, NY, 1997.
|
| [58] |
Nicholas John Pippenger.
Developments in “the synthesis of reliable organisms from unreliable
components”.
Proceedings of Symposia in Pure Mathematics, 50(5), 1990.
(PDF)
|
| [59] |
Todd M. Preuss.
The discovery of cerebral diversity: An unwelcome scientific
revolution.
In Dean Falk and Kathleen R. Gibson, editors, Evolutionary
Anatomy of the Primate Cerebral Cortex, pages 138–164. Cambridge University
Press, 2001.
(PDF)
|
| [60] |
V.S. Ramachandran.
The Tell-Tale Brain: A Neuroscientist’s Quest for What Makes Us
Human.
W. W. Norton, New York, NY, 2011.
|
| [61] |
A. Rodriguez, J. Whitson, and R. Granger.
Derivation and analysis of basic computational operations of
thalamocortical circuits.
Journal of Cognitive Neuroscience, 16:856–877, 2004.
(PDF)
|
| [62] |
A. Saxe, M. Bhand, R. Mudur, B. Suresh, and A. Ng.
Unsupervised learning models of primary cortical receptive fields and
receptive field plasticity.
In J. Shawe-Taylor, R.S. Zemel, P. Bartlett, F. Pereira, and K.Q.
Weinberger, editors, Advances in Neural Information Processing Systems
24. MIT Press, 2011.
(PDF)
|
| [63] |
Thomas E. Schlaepfer, Michael X. Cohen, Caroline Frick, Markus Kosel, Daniela
Brodesser, Nikolai Axmacher, Alexius Young Y. Joe, Martina Kreft, Doris
Lenartz, and Volker Sturm.
Deep brain stimulation to reward circuitry alleviates anhedonia in
refractory major depression.
Neuropsychopharmacology, 33(2):368–377, 2007.
(PDF)
|
| [64] |
K. Semendeferi, E. Armstrong, A. Schleicher, K. Zilles, and G. W. Van Hoesen.
Prefrontal cortex in humans and apes: A comparative study of area 10.
American Journal of Physical Anthropology, 114(3):224–241,
2001.
(PDF)
|
| [65] |
D.A. Seminowicz, H.S. Mayberg, A.R. McIntosh, K. Goldapple, S. Kennedy,
Z. Segal, and S. Rafi-Tari.
Limbic-frontal circuitry in major depression: a path modeling
metanalysis.
NeuroImage, 22(1):409–418, 2004.
(PDF)
|
| [66] |
J. Sepulcre, H. Liu, T. Talukdar, I. Martincorena, and B.T.T. Yeo.
The organization of local and distant functional connectivity in the
human brain.
PLoS Computational Biology, 6(6), 2010.
(PDF)
|
| [67] |
H. Sebastian Seung.
Reading the book of memory: Sparse sampling versus dense mapping of
connectomes.
Neuron, 62(1):17–29, 2009.
(PDF)
|
| [68] |
Michael Shermer.
The Believing Brain: From Ghosts and Gods to Politics and
Conspiracies — How We Construct Beliefs and Reinforce Them as Truths.
Henry Holt and Company, New York, NY, 2011.
|
| [69] |
Raymond Tallis.
Rethinking thinking: How a lumpy bunch of tissue lets us plan,
perceive, calculate, reflect, imagine and exercise free-will.
Wall Street Journal, pages C5–C8, 2011.
(PDF)
|
| [70] |
Henning Tiemeier, Rhoshel K. Lenroot, Deanna K. Greenstein, Lan Tran, Ronald
Pierson, and Jay N. Giedd.
Cerebellum development during childhood and adolescence: A
longitudinal morphometric MRI study.
Neuroimage, 49(1):63–70, 2010.
(PDF)
|
| [71] |
Emanuel Todorov.
Parallels between sensory and motor information processing.
In Michael Gazzaniga, editor, The Cognitive Neurosciences, 4th
Edition, pages 613–624. MIT Press, Cambridge, MA, 2009.
(PDF)
|
| [72] |
J. Tooby and L. Cosmides.
The psychological foundations of culture.
In J. Barkow, L. Cosmides, and J. Tooby, editors, The Adapted
Mind: Evolutionary psychology and the generation of culture. Oxford
University Press, New York, 1992.
(PDF)
|
| [73] |
William R. Uttal.
The New Phrenology: The Limits of Localizing Cognitive Processes
in the Brain.
MIT Press, Cambridge, MA, 2003.
|
| [74] |
Leslie G. Valiant.
A neuroidal architecture for cognitive computation.
Journal of the ACM, 47(5):854–882, 2000.
|
| [75] |
Leslie G. Valiant.
Memorization and association on a realistic neural model.
Neural Compututation, 17(3):527–555, 2005.
(PDF)
|
| [76] |
Leslie G. Valiant, Jianer Chen, and S. Cooper.
Neural computations that support long mixed sequences of knowledge
acquisition tasks.
In Lecture Notes in Computer Science: Theory and Applications of
Models of Computation. Springer, Berlin, 2009.
|
| [77] |
Laurie von Melchner, Sarah L. Pallas, and Mriganka Sur.
Visual behaviour mediated by retinal projections directed to the
auditory pathway.
Nature, 404(6780):871–876, 2000.
(PDF)
|
| [78] |
John von Neumann.
Theory of Self-Reproducing Automata.
University of Illinois Press, Urbana, IL, 1966.
(PDF)
|
| [79] |
Rafael Yuste.
Origin and classification of neocortical interneurons.
Neuron, 48(4):524–527, 2005.
(PDF)
|
1 The term “hominid” refers to both humans and the African apes including Chimps and Bonobos — the descendants of our common ancestor marking the separation of our ancestors from apes in the evolutionary tree. The term “hominin” refers only to humans and our ancestors back to that branching point.
2 O’Reilly [55] reviews models of high-level cognition that depend on the prefrontal cortex and its associated subcortical areas. He suggests that the need to maintain information active in working memory and rapidly update information during inference “appears to be satisfied by bistable activation states and dynamic gating mechanisms”, mechanisms which are “fundamental to digital computers and may be critical for the distinctive aspects of human intelligence.”
3 Terrence Deacon — Anthropology, Evolutionary Biology, Neuroscience at Berkeley — comments that “[e]xplaining the extravagant complexity of the human language and our competence to acquire it has long posed challenges for natural selection theory. [...] Recent [evolutionary-development] approaches have identified developmental processes that help to explain how complex functional synergies can evolve by Darwinian means. Interestingly, many of these developmental mechanisms bear a resemblance to aspects of Darwin’s mechanism of natural selection, often differing only in one respect (e.g., form of duplication, kind of variation, competition/cooperation). A common feature is an interplay between processes of stabilizing selection and processes of relaxed selection at different levels of organism function. These may play important roles in the many levels of evolutionary process contributing to language. Surprisingly, the relaxation of selection at the organism level may have been a source of many complex synergistic features of the human language capacity, and may help explain why so much language information is ‘inherited’ socially.” — see Proceedings of the National Academy of Science, 2010 May 11; 107(2):9000-9006.
4 See Balsters et al [3] for an interesting study showing that “interconnected brain areas [superficially, the parts of the cerebellum connected to the prefrontal cortex and the cerebellar lobules connected to the motor cortex] evolve in tandem because evolutionary pressures act on complete functional systems rather than on individual brain areas.”