The overall approach to developing FFCM involves constrained global optimization and uncertainty quantification/minimization following a careful rate evaluation of the literature kinetic data. The figure below outlines the overall approach:
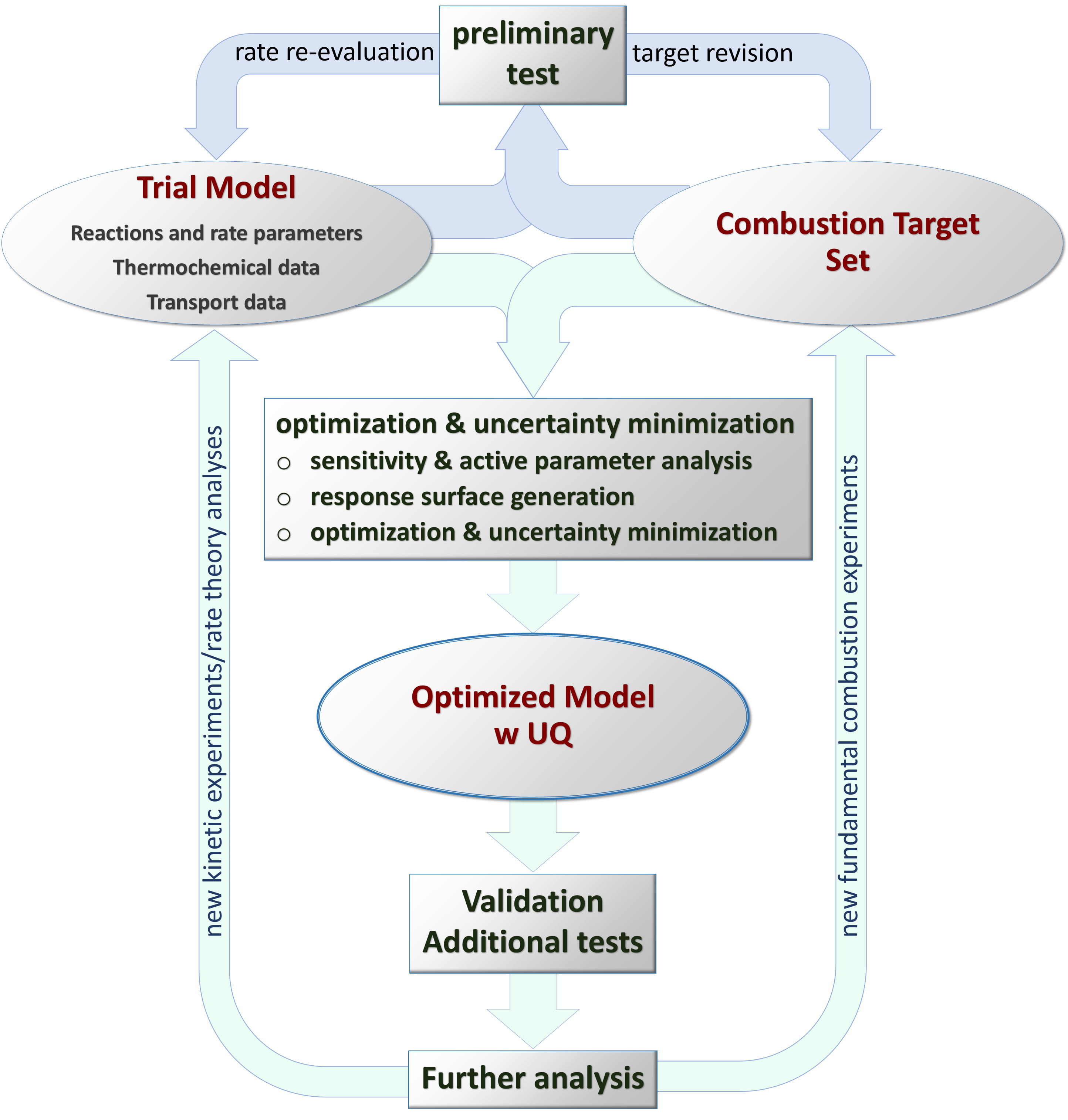
1) A trial reaction model is compiled along with its associated thermochemical and transport databases. Rate evaluation relies mostly on experimental and theoretical rate coefficients. In addition to the nominal rate expression, the uncertainty factor of the rate expression is also estimated from existing data. Therefore, the reaction model is comprised of a list of elementary reactions, their associated rate expressions, and the uncertainty factors.
2) Fundamental combustion property data are compiled for the target fuels/species. The resulting database is called the target dataset. Relevant properties include the global combustion responses, including the laminar flame speed and shock tube ignition delay times, and some detailed time-history profiles of key species in a fuel oxidation process. Fairly rigorous uncertainty analysis is carried out for each dataset. Specific target condition and target value are chosen to best represent the thermodynamic condition range of each data set.
3) The trial model is subject to a preliminary test against the target dataset. Ensuing sensitivity analyses help to identify certain problems in the trial model. For example, after an initial screening test, it was determined that the model uncertainty can be reduced significantly with respect to the laminar flame speed of H2/air mixtures if the uncertainty in the rate coefficient of the reaction
H2 + OH ⟷ H2O + H
can be reduced to the ±20% level. Hanson's group' at Stanford subsequently made the measurement on the reaction [1] with a 2σ uncertainty of ±17%. The rate measurement suggests that the higher end values within the uncertainty band of the measured H2/air laminar flame speed are probably more accurate than the lower values. Preliminary tests also include forward uncertainty quantification (UQ). The quality of the current kinetic rate knowledge is accessed in its predictive precision against the target dataset.
4) Global constrained optimization and model uncertainty minimization followed. This is done in several steps.
·
Sensitivity
analysis of a particular combustion target with respect to the reaction rate
coefficient (the A factor).
·
Active rate parameters are chosen based on ranked
sensitivity x uncertainty spectrum of each target.
·
Response surfaces are built through computational
experiments using an array of methods.
·
Optimization and
uncertainty minimization using the Method of
Uncertainty Minimization by Polynomial Chaos Expansion (MUM-PCE), resulting in an
optimized reaction model and the covariance matrix that describes the joint
probability distribution function of the active rate parameters.
5) The optimized model is tested against available experimental data, including validation against both the target dataset and test against other available combustion property data.
6) Further analyses of the model and the target dataset reveal key uncertainty in several aspects of the problems, including the rate coefficient and reaction pathways and the combustion property data. Recommendations are made and documented on the basis of these analyses.
Reference
[1] Lam KY, Davidson DF, Hanson RK. A shock tube study of H2+ OH → H2O+ H using OH laser absorption. Int J Chem Kinet. 2013;45:363-73.




